有机化学 ›› 2022, Vol. 42 ›› Issue (1): 226-234.DOI: 10.6023/cjoc202107045 上一篇 下一篇
研究论文
冯易浇a, 何静a, 韦玥婷a, 汤婷b, 李春天a,*( ), 刘平a,*(
), 刘平a,*( )
)
收稿日期:2021-07-21
修回日期:2021-08-24
发布日期:2021-09-08
通讯作者:
李春天, 刘平
基金资助:
Yijiao Fenga, Jing Hea, Yueting Weia, Ting Tangb, Chuntian Lia( ), Ping Liua(
), Ping Liua( )
)
Received:2021-07-21
Revised:2021-08-24
Published:2021-09-08
Contact:
Chuntian Li, Ping Liu
Supported by:文章分享
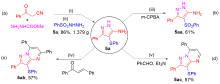
建立了3-氧代-3-芳基丙腈、肼基甲酸甲酯(或水合肼)和芳基磺酰肼的一锅两步反应. 在I2和N-碘代丁二酰亚胺(NIS)的作用下, 通过环化、磺基化和脱酯基化反应构建了一系列3-芳基-4-(芳硫基)-1H-吡唑-5-胺化合物. 该方法具有良好的原子经济性、温和的反应条件、广泛的底物适用范围和克级规模的合成. 此外, 还对3-芳基-4-(芳硫基)-1H-吡唑-5-胺产物的进一步转化进行了研究.
冯易浇, 何静, 韦玥婷, 汤婷, 李春天, 刘平. 一锅两步策略高效合成3-芳基-4-(芳硫基)-1H-吡唑-5-胺衍生物[J]. 有机化学, 2022, 42(1): 226-234.
Yijiao Feng, Jing He, Yueting Wei, Ting Tang, Chuntian Li, Ping Liu. One-Pot Two-Step Strategy for Efficient Synthesis of 3-Aryl-4-(arylthio)-1H-pyrazol-5-amines Derivatives[J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 226-234.
| Entry | Catalyst (mol%) | Solvent/mL | Yieldb/% |
|---|---|---|---|
| 1 | — | EtOH | Trace |
| 2 | NIS (50) | EtOH | 84 |
| 3 | NIS (20) | EtOH | 85 |
| 4 | NIS (10) | EtOH | 86 |
| 5 | NIS (5) | EtOH | 67 |
| 6 | I2 (10) | EtOH | 97 |
| 7 | I2 (5) | EtOH | 92 |
| 8 | TBAI (10) | EtOH | Trace |
| 9 | I2 (10) | 1,4-Dixoane | 57 |
| 10 | I2 (10) | DCE | 64 |
| 11 | I2 (10) | DMSO | 45 |
| 12 | I2 (10) | DMF | 34 |
| 13 | I2 (10) | THF | 56 |
| 14c | I2 (10) | EtOH | 85 |
| Entry | Catalyst (mol%) | Solvent/mL | Yieldb/% |
|---|---|---|---|
| 1 | — | EtOH | Trace |
| 2 | NIS (50) | EtOH | 84 |
| 3 | NIS (20) | EtOH | 85 |
| 4 | NIS (10) | EtOH | 86 |
| 5 | NIS (5) | EtOH | 67 |
| 6 | I2 (10) | EtOH | 97 |
| 7 | I2 (5) | EtOH | 92 |
| 8 | TBAI (10) | EtOH | Trace |
| 9 | I2 (10) | 1,4-Dixoane | 57 |
| 10 | I2 (10) | DCE | 64 |
| 11 | I2 (10) | DMSO | 45 |
| 12 | I2 (10) | DMF | 34 |
| 13 | I2 (10) | THF | 56 |
| 14c | I2 (10) | EtOH | 85 |
| Entry | [I] source (mol%) | T/℃ | Yieldb/% |
|---|---|---|---|
| 1 | — | 80 | 19 |
| 2 | I2 (50) | 80 | 78 |
| 3 | KI (50) | 80 | 54 |
| 4 | NH4I (50) | 80 | 46 |
| 5 | TBAI (50) | 80 | 38 |
| 6 | NIS (50) | 80 | 88 |
| 7 | NIS (30) | 80 | 82 |
| 8 | NIS (70) | 80 | 87 |
| 9 | NIS (50) | 60 | 30 |
| 10 | NIS (50) | 100 | 75 |
| 11c | NIS (50) | 80 | 94 |
| 12d | NIS (50) | 80 | 70 |
| Entry | [I] source (mol%) | T/℃ | Yieldb/% |
|---|---|---|---|
| 1 | — | 80 | 19 |
| 2 | I2 (50) | 80 | 78 |
| 3 | KI (50) | 80 | 54 |
| 4 | NH4I (50) | 80 | 46 |
| 5 | TBAI (50) | 80 | 38 |
| 6 | NIS (50) | 80 | 88 |
| 7 | NIS (30) | 80 | 82 |
| 8 | NIS (70) | 80 | 87 |
| 9 | NIS (50) | 60 | 30 |
| 10 | NIS (50) | 100 | 75 |
| 11c | NIS (50) | 80 | 94 |
| 12d | NIS (50) | 80 | 70 |

| [1] |
Daidone, G.; Maggio, B.; Plescia, S.; Raffa, D.; Musiu, C.; Milia, C.; Perra, G.; Marongiu, M. E. Eur. J. Med. Chem. 1998, 33, 375.
doi: 10.1016/S0223-5234(98)80004-4 |
| [2] |
Penning, T. D.; Talley J. J.; S. R. Bertenshaw, S. R.; J. S. Carter, J. S.; Collins, P. R.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. S.; Yu, S. S.; Anderson, G. G.; Burton, E. G.; Cogburn, J. N.; S. A. Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347.
pmid: 9135032 |
| [3] |
Elvin, L. A.; John, E. C.; Leon, C. G.; John, J. L.; Harry, E. R. J. Med. Chem. 1964, 7, 259.
doi: 10.1021/jm00333a004 |
| [4] |
Fancelli, D.; Berta, D.; Bindi, S.; Cameron, A.; Cappella, P.; Carpinelli, P.; Catana, C.; Forte, B.; Giordano, P.; Giorgini, M. L.; Mantegani, S.; Marsiglio, A.; Meroni, M.; Moll, J.; Pittala, V.; Roletto, F.; Severino, D.; Soncini, C.; Storici, P.; Tonani, R.; Varasi, M.; Vulpetti, A.; Vianello, P. J. Med. Chem. 2005, 48, 3080.
pmid: 15828847 |
| [5] |
(a) Pevarello, P.; Brasca, M. G.; Orsini, P.; Traquandi, G.; Longo, A.; Nesi, M.; Orzi, F.; Piutti, C.; Sansonna, P.; Varassi, M.; Cameron, A.; Vulpetti, A.; Roletto, F.; Alzani, R.; Ciomei, M.; Alanese, C.; Pastori, W.; Marsiglio, A.; Pesenti, E.; Fiorentini, F.; Bischoff, R.; Mercurio, C. J. Med. Chem. 2005, 48, 2944.
pmid: 15828833 |
|
(b) Kuma, Y.; Sabio, G.; Bain, J.; Shpiro, N.; Márquez, R.; Cuenda, A. J. Biol. Chem. 2005, 280, 19472.
doi: 10.1074/jbc.M414221200 pmid: 15828833 |
|
| [6] |
Bagley, M. C.; Davis, T.; Dix, M. C.; Widdowson, M. C.; Kipling, D. Org. Biomol. Chem. 2006, 4, 4158.
pmid: 17312972 |
| [7] |
Dong, J. J.; Li, Q. S.; Wang, S. F.; Li, C. Y.; Zhao, X.; Qiu, H. Y.; Zhao, M. Y.; Zhu, H. L. Org. Biomol. Chem. 2013, 11, 6328.
doi: 10.1039/c3ob40776d |
| [8] |
Wang, S. F.; Yin, Y.; Zhang, Y. L.; Mi, S. W.; Zhao, M. Y.; Lv, P. C.; Wang; B. Z.; Zhu, H. L. Eur. J. Med. Chem. 2015, 93, 291.
doi: 10.1016/j.ejmech.2015.02.018 |
| [9] |
(a) Allah, A. G. G.; Hefny, M. M.; Salih, El-Basiouny, M. S. Corrosion 1989, 45, 574.
doi: 10.5006/1.3577875 |
|
(b) Allah, A. G.; Badawy, M. W.; Reham, H. H.; Abou-Romia, M. M. J. Appl. Electrochem. 1989, 19, 928.
doi: 10.1007/BF01007942 |
|
|
(c) Badawy, W. A.; Hefny, M. M.; El-Egamy, S. S. Corrosion 1990, 46, 978.
doi: 10.5006/1.3585055 |
|
|
(d) Abou-Romia, M. M.; Abd El-Rahaman, H. A.; El-Sayed, H. A. M. Bull. Electrochem. 1990, 6, 757.
|
|
| [10] |
Joshi, K. C.; Pathak, V. N.; Garg, U J. Heterocycl. Chem. 1979, 16, 1141.
doi: 10.1002/jhet.v16:6 |
| [11] |
Hanefeld, U.; Rees, C. W.; White, A. J. P. J. Chem. Soc., Perkin Trans. 1996, 1, 1545.
|
| [12] |
(a) Dodd, D. S.; Martinez, R. L.; Kamau, M.; Ruam, Z.; Kirk, K. V.; Cooper, C. B.; Hermsmeier, M. A.; Traeger, S. C., Poss, M. A.; J. Comb. Chem. 2005, 7, 584.
doi: 10.1021/cc049814s |
|
(b) Villemin, D.; Benalloum, A. Synth. Commun. 1991, 21, 1.
doi: 10.1080/00397919108020783 |
|
| [13] |
Ioannidou, H. A.; Koutentis, P. A. Tetrahedron 2009, 65, 7023.
doi: 10.1016/j.tet.2009.06.041 |
| [14] |
Bagley, M. C.; Davis, T.; Dix, M. C.; Widdowson, C. S.; Kipling, D. Org. Biomol. Chem. 2006, 4, 4158.
pmid: 17312972 |
| [15] |
Su, W. N.; Lin, T. P.; Cheng, K. M.; Sung, K. C.; Lin, S. K.; Wong, F. F. J. Heterocycl. Chem. 2010, 47, 831.
doi: 10.1002/jhet.343 |
| [16] |
Kim, B. R.; Sung, G. H.; Ryu, K. E.; Lee, S. G.; Yoon, H. J.; Shin, D. S.; Yoon, Y. J. Chem. Commun. 2015, 51, 9201.
doi: 10.1039/C5CC02020D |
| [17] |
Zora, M.; Kivrak, A. J. Org. Chem. 2011, 76, 9379.
doi: 10.1021/jo201685p |
| [18] |
Reddy, G. J.; Latha, D.; Rao, K. S. Org. Prep. Proced. Int. 2004, 36, 494.
doi: 10.1080/00304940409356638 |
| [19] |
Kirkham, J. D.; Edeson, S. J.; Stokes, S.; Harrity, J. P. Org. Lett. 2012, 14, 5354.
doi: 10.1021/ol302418b pmid: 23025502 |
| [20] |
Senadi, G. C.; Hu, W. P.; Lu, T. Y.; Garkhedkar, A. M.; Vandavasi, J. K.; Wang, J. J. Org. Lett. 2015, 17, 1521.
doi: 10.1021/acs.orglett.5b00398 |
| [21] |
Ma, C.; Wen, P.; Li, J.; Han, X.; Wu, Z.; Huang, G. Adv. Synth. Catal. 2016, 358, 1073.
doi: 10.1002/adsc.201500767 |
| [22] |
(a) Suryakiran, N.; Reddy, T. S.; Latha, K. A.; Prabhakar, P.; Yadagiri, K.; Venkateswarlu, Y. J. Mol. Catal. A: Chem. 2006, 258, 371.
doi: 10.1016/j.molcata.2006.07.054 pmid: 29977390 |
|
(b) Suryakiran, N.; Ramesh, D.; Venkateswarlu, Y. Green Chem. Lett. Rev. 2007, 1, 73.
doi: 10.1080/17518250701771909 pmid: 29977390 |
|
|
(c) Everson, N.; Yniguez, K.; Loop, L.; Lazaro, H.; Belanger, B.; Koch, G.; Bach, J.; Manjunath, A.; Schioldager, R.; Law, J.; Grabenauer, M.; Eagon, S. Tetrahedron Lett. 2019, 60, 72.
doi: 10.1016/j.tetlet.2018.11.060 pmid: 29977390 |
|
|
(d) Kelada, M.; Walsh, J. M. D.; Devine, R. W.; McArdle, P.; Stephens, J. C. Beilstein J. Org. Chem. 2018, 14, 1222.
doi: 10.3762/bjoc.14.104 pmid: 29977390 |
|
| [23] |
(a) Shaabani, A.; Nazeri, M. T.; Afshari, R. Mol. Diversity 2019, 23, 751.
doi: 10.1007/s11030-018-9902-8 |
|
(b) Sun, K.; Li, G.; Li, Y.; Yu, J.; Zhao, Q.; Zhang, Z.; Zhang, G. Adv. Synth. Catal. 2020, 362, 1947.
doi: 10.1002/adsc.v362.10 |
|
| [24] |
Wang, P.; Xie, Z.; Hong, Z.; Tang, J.; Wong, O.; Lee, C. S.; Wong, N.; Lee, S. J. Mater. Chem. 2003, 13, 1894.
doi: 10.1039/b302972g |
| [25] |
Fan, W.; Ye, Q.; Xu, H. W.; Jiang, B.; Wang, S. L.; Tu, S. J. Org. Lett. 2013, 15, 2258.
doi: 10.1021/ol4008266 |
| [26] |
Jiang, B.; Fan, W.; Sun, M. Y.; Ye, Q.; Wang, S. L.; Tu, S. J.; Li, G. J. Org. Chem. 2014, 79, 5258.
doi: 10.1021/jo500823z pmid: 24833111 |
| [27] |
Tu, X. J.; Hao, W. J.; Ye, Q.; Wang, S. S.; Jiang, B.; Li, G.; Tu, S. J. J. Org. Chem. 2014, 79, 11110.
doi: 10.1021/jo502096t |
| [28] |
Simpkins, N. S. In Sulfones in Organic Synthesis, Ed.: Baldwin, J. E., Pergamon Press, Oxford, UK, 1993.
|
| [29] |
Asai, T.; Takeuchi, T.; Diffenderfer, J.; Sibley, D. L. Antimicrob. Agents Chemother.. 2002, 46, 2393.
doi: 10.1128/AAC.46.8.2393-2399.2002 |
| [30] |
(a) Caddick, S.; Aboutayab, K.; West, R. Synlett 1993, 231.
pmid: 30129769 |
|
(b) Wei, W.; Bao, P.; Yue, H.; Liu, S.; Wang, L.; Li, Y.; Yang, D. Org. Lett. 2018, 20, 5291.
doi: 10.1021/acs.orglett.8b02231 pmid: 30129769 |
|
|
(c) Sun, P.; Yang, D.; Wei, W.; Jiang, M.; Wang, Z.; Zhang, L.; Zhang, H.; Zhang, Z.; Wang, Y.; Wang, H. Green Chem. 2017, 19, 4785.
doi: 10.1039/C7GC01891F pmid: 30129769 |
|
| [31] |
Ragno, R.; Coluccia, A.; La Regina, G.; De Martino, G.; Piscitelli, F.; Lavecchia, A.; Novellino, E.; Bergamini, A.; Ciaprini, C.; Sinistro, A.; Maga, G.; Crespan, E.; Artico, M.; Silvestri, R. J. Med. Chem. 2006, 49, 3172.
pmid: 16722636 |
| [32] |
Silvestri, R.; De Martino, G.; La Regina, G.; Artico, M.; Massa, S.; Vargiu, L.; Mura, M.; Loi, A. G.; Marceddu, T.; La Colla, P. J. Med. Chem. 2003, 46, 2482.
pmid: 12773052 |
| [33] |
Avis, I.; Martínez, A.; Tauler, J.; Zudaire, E.; Mayburd, A.; Abu- Ghazaleh, R.; Ondrey, F.; Mulshine, J. L. Cancer Res. 2005, 65, 4181.
doi: 10.1158/0008-5472.CAN-04-3441 |
| [34] |
De Martino, G.; La Regina, G.; Coluccia, A.; Edler, M. C.; Barbera, M. C.; Brancale, A.; Wilcox, E.; Hamel, E.; Artico, M.; Silvestri, R. J. Med. Chem. 2004, 47, 6120.
doi: 10.1021/jm049360d |
| [35] |
Funk, C. D. Nat. Rev. Drug Discovery 2005, 4, 664.
doi: 10.1038/nrd1796 |
| [36] |
Kaneda, K.; Mitsudome, T. Chem. Rec. 2017, 17, 1.
doi: 10.1002/tcr.201780101 |
| [37] |
Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T. A.; Liu, X. Chem. Soc. Rev. 2015, 44, 291.
doi: 10.1039/C4CS00239C |
| [38] |
Qiu, G.; Zhou, K.; Wu, J. Chem. Commun. 2018, 54, 12561.
doi: 10.1039/C8CC07434H |
| [39] |
Dong, D. Q.; Hao, S. H.; Yang, D. S.; Li, L. X.; Wang, Z. L. Eur. J. Org. Chem. 2017, 45, 6576.
|
|
(a) Liu, Y.; Xiong, J.; Wei, L. Chin. J. Org. Chem. 2017, 37, 1667. (in Chinese)
doi: 10.6023/cjoc201702009 |
|
|
(刘云云, 熊进, 韦丽, 有机化学, 2017, 37, 1667.)
doi: 10.6023/cjoc201702009 |
|
|
(b) Xu, X. M.; Chen, D. M.; Wang, Z. L. Chin. J. Org. Chem. 2019, 39, 3338. (in Chinese)
doi: 10.6023/cjoc201904068 |
|
|
(徐鑫明, 陈德茂, 王祖利, 有机化学, 2019, 39, 3338.)
doi: 10.6023/cjoc201904068 |
|
|
(c) Chen, S. H.; Wang, M.; Jiang, X. F. Acta Phys.-Chim. Sin. 2019, 35, 954. (in Chinese)
doi: 10.3866/PKU.WHXB201810044 |
|
|
(陈世豪, 王明, 姜雪峰, 物理化学学报, 2019, 35, 954.)
|
|
|
(d) Liu, Y. Y.; Xiong, J.; Wei, L. Chin. J. Org. Chem. 2017, 37, 1667. (in Chinese)
doi: 10.6023/cjoc201702009 |
|
|
(刘云云, 熊进, 韦丽, 有机化学, 2017, 37, 1667.)
doi: 10.6023/cjoc201702009 |
|
|
(e) Xu, X. M.; Yang, H. L.; Li, W. Z. Chin. J. Org. Chem. 2020, 40, 1912. (in Chinese)
doi: 10.6023/cjoc201912044 |
|
|
(徐鑫明, 杨翰林, 李文忠, 有机化学, 2020, 40, 1912.)
doi: 10.6023/cjoc201912044 |
|
|
(f) Xu, X. M.; Li, J. Z.; Wang, Z. L. Chin. J. Org. Chem. 2020, 40, 886. (in Chinese)
doi: 10.6023/cjoc201910020 |
|
|
(徐鑫明, 李家柱, 王祖利, 有机化学, 2020, 40, 886.)
doi: 10.6023/cjoc201910020 |
|
|
(g) Li, Y.; Wan, J. Chin. J. Org. Chem. 2020, 40, 3889. (in Chinese)
doi: 10.6023/cjoc202005026 |
|
|
(李毅, 万结平, 有机化学, 2020, 40, 3889.)
doi: 10.6023/cjoc202005026 |
|
| [40] |
(a) Wang, L.; Zhang, M.; Zhang, Y.; Liu, Q.; Zhao, X.; Li, J.; Luo, Z.; Wei, W. Chin. Chem. Lett. 2020, 31, 67.
doi: 10.1016/j.cclet.2019.05.041 |
|
(b) Liu, Q.; Lv, Y.; Liu, R.; Zhao, X.; Wang, J.; Wei, W. Chin. Chem. Lett. 2021, 32, 136.
doi: 10.1016/j.cclet.2020.11.059 |
|
|
(a) Armstrong, R. W.; Combs, A. P.; Tempest, P. A.; Brown, S. D.; Keating, T. A. Acc. Chem. Res. 1996, 29, 123.
doi: 10.1021/ar9502083 |
|
|
(b) Dömling, A.; Ugi, I. Angew. Chem., nt. Ed. 2000, 39, 3168.
|
|
| [41] |
(a) Meng, N.; Lv, Y.; Liu, Q.; Liu, R.; Zhao, X.; Wei, W. Chin. Chem. Lett. 2021, 32, 258.
doi: 10.1016/j.cclet.2020.11.034 |
|
(b) Gui, Q. W.; Wang, B. B.; Zhu, S.; Li, F. L., Zhu, M. X..; Yi, M.; Yu, J. L.; Wu, Z.-L.; He, W. M. Green Chem. 2021, 23, 4430.
doi: 10.1039/D1GC01017D |
|
|
(c) Wu, Y.; Chen, J.Y.; Ning, J.; Jiang, X.; Deng, J.; Deng, Y.; Xu, R.; He, W. M. Green Chem. 2021, 23, 3950.
doi: 10.1039/D1GC00562F |
|
|
(d) Wang, S. C.; Liu, P. Y.; Chen, Y. X.; Shen, Z. J.; Hao, W. J.; Tu, S. J.; Jiang, B. Chem. Commun. 2021, 57, 7966.
doi: 10.1039/D1CC02973H |
|
|
(e) Li, Q. X.; Li, M. W.; Shi, S. Q.; Ji, X. S.; He, C. L.; Jiang, B.; Hao, W. J. Chin. J. Org. Chem. 2020, 40, 384. (in Chinese)
doi: 10.6023/cjoc201909041 |
|
|
(李庆雪, 李梦伟, 时绍青, 季晓霜, 何春兰, 姜波, 郝文娟, 有机化学, 2020, 40, 384.)
doi: 10.6023/cjoc201909041 |
|
|
(f) Zhang, T. S.; Hao, W. J.; Wang, R.; Wang, S. C.; Tu, S. J.; Jiang, B. Green Chem. 2020, 22, 4259.
doi: 10.1039/D0GC00771D |
|
| [42] |
Sun, J.; Qiu, J. K.; Zhu, Y. L.; Guo, C.; Hao, W. J.; Jiang, B.; Tu, S. J. J. Org. Chem. 2015, 80, 8217.
doi: 10.1021/acs.joc.5b01280 |
| [43] |
Sun, J.; Qiu, J. K.; Jiang, B.; Hao, W. J.; Guo, C.; Tu, S. J. J. Org. Chem. 2016, 81, 3321.
doi: 10.1021/acs.joc.6b00332 |
| [44] |
Wei, Y.; Liu, P.; Liu, Y.; He, J.; Li, X.; Li, S.; Zhao, J. Org. Biomol. Chem. 2021, 19, 3932.
doi: 10.1039/D1OB00438G |
| [45] |
(a) Chen, L. J.; Zhang, J.; Wei, Y. T.; Yang, Z.; Liu, P.; Zhang, J; Dai, B. Tetrahedron 2019, 75, 130664.
doi: 10.1016/j.tet.2019.130664 |
|
(b) Wei, Y. T.; He, J.; Liu, Y. L.; Xu, L.; Vaccaro, L.; Liu, P.; Gu, Y. L. ACS Omega 2020, 5, 18515.
doi: 10.1021/acsomega.0c02590 |
|
|
(c) Wei, Y. T.; Liu, Y. L.; He, J.; Li, X. Z.; Liu, P.; Zhang, J. Tetrahedron 2020, 76, 131646.
doi: 10.1016/j.tet.2020.131646 |
|
|
(d) He, J.; Wei, Y. T.; Li, X. Z.; Dai, B.; Liu, P. Synthesis 2022, 54, 490.
doi: 10.1055/a-1592-6394 |
|
| [46] |
(a) Yang, F. L.; Wang, F. X.; Wang, T. T.; Wang, Y. J.; Tian, S. K. Chem. Commun. 2014, 50, 2111.
doi: 10.1039/c3cc48961b |
|
(b) Yang, F. L.; Tian, S. K. Angew. Chem., Int. Ed. 2013, 52, 1.
|
|
| [47] |
Yang, F. L.; Yang, G.; Yu, B. K.; Jin, Y. X.; Tian, S. K. Adv. Synth. Catal. 2016, 358, 3368.
doi: 10.1002/adsc.v358.21 |
| [48] |
Zhang, J.; Li, W.; Liu, Y.; Liu, P. ChemistrySelect 2020, 5, 5497.
doi: 10.1002/slct.v5.19 |
| [49] |
Talla, A.; Driessen, B.; Straathof, N. J.; Milroy, L. G.; Brunsveld, L.; Hessel, V.; Noel, T. Adv. Synth. Catal, 2015, 357, 2180.
doi: 10.1002/adsc.v357.10 |
| [50] |
Lv, Y.; Luo, J.; Ma, Y.; Dong, Q.; He, L. Org. Chem. Front. 2021, 8, 2461.
doi: 10.1039/D1QO00112D |
| [1] | 高宝昌, 石雨, 田媛, 张治国, 张婧如, 孙宇峰, 毛国梁, 戴凌燕. 4-甲基-2-氧代-6-芳氨基-二氢-吡喃-3-腈衍生物的合成[J]. 有机化学, 2024, 44(2): 644-649. |
| [2] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [3] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [4] | 樊思捷, 董武恒, 梁彩云, 王贵超, 袁瑶, 尹作栋, 张兆国. 可见光诱导的自由基环化反应构建4-芳基-1,2-二氢萘类化合物[J]. 有机化学, 2023, 43(9): 3277-3286. |
| [5] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [6] | 冯莹珂, 王贺, 崔梦行, 孙然, 王欣, 陈阳, 李蕾. 可见光诱导的新型官能化芳基异腈化合物的二氟烷基化环化反应[J]. 有机化学, 2023, 43(8): 2913-2925. |
| [7] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [8] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [9] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [10] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [11] | 蔡荣斌, 李冰, 周琪, 朱隆懿, 罗军. 4,8,9,10-四官能化的2-氮杂金刚烷及其2-氮杂原金刚烷骨架异构体的合成[J]. 有机化学, 2023, 43(6): 2217-2225. |
| [12] | 孔德亮, 戴闻, 赵怡玲, 陈艺林, 朱红平. 脒基胺硼基硅宾与单酮和二酮的氧化环加成反应研究[J]. 有机化学, 2023, 43(5): 1843-1851. |
| [13] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [14] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [15] | 汤振, 皮超, 吴养洁, 崔秀灵. 铑催化2-芳基-2H-吲唑与硫叶立德的酰甲基化/串联环化反应高效构建6-芳基吲唑并[2,3-a]喹啉类衍生物[J]. 有机化学, 2023, 43(3): 1187-1196. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||