有机化学 ›› 2023, Vol. 43 ›› Issue (6): 2110-2119.DOI: 10.6023/cjoc202208010 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑
研究论文
陆晓雨*( ), 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅
), 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅
收稿日期:2022-08-08
修回日期:2022-11-01
发布日期:2023-01-11
基金资助:
Xiaoyu Lu*( ), Xiaomei Sun, Yaqing Niu, Junchao Wang, Wenjing Yin, Mengting Gao, Zi Liu, Zhenghuan Wei, Tinghua Tao
), Xiaomei Sun, Yaqing Niu, Junchao Wang, Wenjing Yin, Mengting Gao, Zi Liu, Zhenghuan Wei, Tinghua Tao
Received:2022-08-08
Revised:2022-11-01
Published:2023-01-11
Contact:
E-mail: Supported by:文章分享
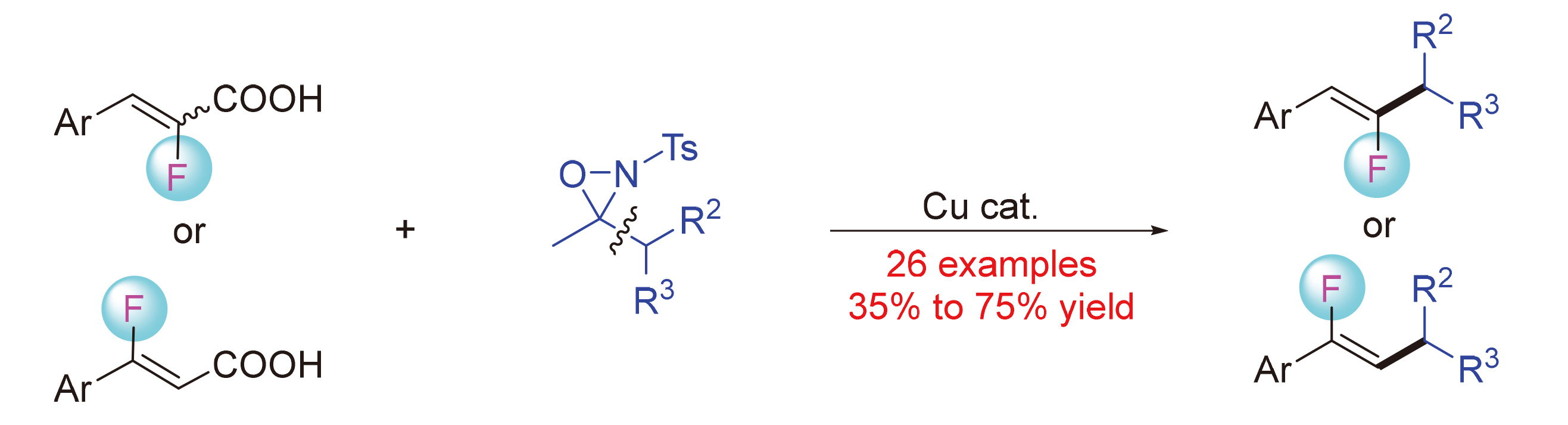
报道了铜催化氟代丙烯酸与N-甲苯磺酰氧杂吖丙啶的脱羧偶联反应. 一系列含多种官能团的氟代丙烯酸均是兼容的反应底物. 一级、二级以及三级烷基取代的吖丙啶杂环都可以顺利地参与反应, 以中等以上的收率给出期望的单氟烯烃. 该脱羧反应展现出良好的官能团兼容性及卓越的立体选择性, 为在医药科学和材料科学上有着重要应用价值的单氟烯烃提供了一种新颖、实用的合成策略. 除了α-氟代肉桂酸, β-氟代肉桂酸也可以作为反应底物. 该方法可为多种取代类型的单氟烯烃提供合成途径, 该反应也为活性分子的后期修饰提供了策略.
陆晓雨, 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅. 铜催化氟代丙烯酸与氧杂吖丙啶的脱羧交叉偶联反应[J]. 有机化学, 2023, 43(6): 2110-2119.
Xiaoyu Lu, Xiaomei Sun, Yaqing Niu, Junchao Wang, Wenjing Yin, Mengting Gao, Zi Liu, Zhenghuan Wei, Tinghua Tao. Copper-Catalyzed Decarboxylative Cross-Coupling of α‑Fluoroacrylic Acids with N-Tosyl Oxaziridines[J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2110-2119.
| Entrya | Catalyst | Ligand | Solvent | Yield/% (Z:E>30:1) |
|---|---|---|---|---|
| 1 | CuI | L1 | DCE | 37 |
| 2 | CuBr | L1 | DCE | 26 |
| 3 | CuCl | L1 | DCE | 25 |
| 4 | CuTc | L1 | DCE | 55 |
| 5 | Cu2O | L1 | DCE | 62 |
| 6 | CuOTf.Ph | L1 | DCE | 40 |
| 7 | Cu(CH3CN)4PF6 | L1 | DCE | 78 |
| 8 | Fe(OAc)2 | L1 | DCE | 35 |
| 9 | Cu(OTf)2 | L1 | DCE | 42 |
| 10 | Cu(acac)2 | L1 | DCE | 72 |
| 11 | CuO | L1 | DCE | 28 |
| 12 | CuBr2 | L1 | DCE | 29 |
| 13 | Cu(OAc)2 | L1 | DCE | 31 |
| 14 | Cu(CH3CN)4PF6 | L2 | DCE | 37 |
| 15 | Cu(CH3CN)4PF6 | L3 | DCE | 22 |
| 16 | Cu(CH3CN)4PF6 | L4 | DCE | 18 |
| 17 | Cu(CH3CN)4PF6 | L1 | PhF | 45 |
| 18 | Cu(CH3CN)4PF6 | L1 | PhCl | 56 |
| 19 | Cu(CH3CN)4PF6 | L1 | PhH | 43 |
| 20 | Cu(CH3CN)4PF6 | L1 | PhH/DCE (V:V=1:1) | 38 |
| 21 | Cu(CH3CN)4PF6 | L1 | DCE | 69 |
| 22 | Cu(CH3CN)4PF6 | L1 | DCE | 65 |
| 23 | — | L1 | DCE | Trace |
| Entrya | Catalyst | Ligand | Solvent | Yield/% (Z:E>30:1) |
|---|---|---|---|---|
| 1 | CuI | L1 | DCE | 37 |
| 2 | CuBr | L1 | DCE | 26 |
| 3 | CuCl | L1 | DCE | 25 |
| 4 | CuTc | L1 | DCE | 55 |
| 5 | Cu2O | L1 | DCE | 62 |
| 6 | CuOTf.Ph | L1 | DCE | 40 |
| 7 | Cu(CH3CN)4PF6 | L1 | DCE | 78 |
| 8 | Fe(OAc)2 | L1 | DCE | 35 |
| 9 | Cu(OTf)2 | L1 | DCE | 42 |
| 10 | Cu(acac)2 | L1 | DCE | 72 |
| 11 | CuO | L1 | DCE | 28 |
| 12 | CuBr2 | L1 | DCE | 29 |
| 13 | Cu(OAc)2 | L1 | DCE | 31 |
| 14 | Cu(CH3CN)4PF6 | L2 | DCE | 37 |
| 15 | Cu(CH3CN)4PF6 | L3 | DCE | 22 |
| 16 | Cu(CH3CN)4PF6 | L4 | DCE | 18 |
| 17 | Cu(CH3CN)4PF6 | L1 | PhF | 45 |
| 18 | Cu(CH3CN)4PF6 | L1 | PhCl | 56 |
| 19 | Cu(CH3CN)4PF6 | L1 | PhH | 43 |
| 20 | Cu(CH3CN)4PF6 | L1 | PhH/DCE (V:V=1:1) | 38 |
| 21 | Cu(CH3CN)4PF6 | L1 | DCE | 69 |
| 22 | Cu(CH3CN)4PF6 | L1 | DCE | 65 |
| 23 | — | L1 | DCE | Trace |
| [1] |
(a) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
doi: 10.1021/jm800219f |
|
(b) Wang, J.; SánchezRoselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
doi: 10.1021/cr4002879 |
|
|
(c) Liu, Q.; Ni, C.; Hu, J. Natl. Sci. Rev. 2017, 4, 303.
doi: 10.1093/nsr/nwx058 |
|
|
(d) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C |
|
|
(d) Liao, F.; Yu, J; Zhou, J. Chin. J. Org. Chem. 2017, 37, 2175. (in Chinese)
doi: 10.6023/cjoc201705001 |
|
|
(廖富民, 余金生, 周剑, 有机化学, 2017, 37, 2175.)
doi: 10.6023/cjoc201705001 |
|
|
(e) He, S.; Pi, J.; Li, Y.; Lu, X.; Fu, Y. Acta Chim. Sinica. 2018, 76, 956. (in Chinese)
doi: 10.6023/A18080333 |
|
|
(何世江, 皮静静, 李炎, 陆熹, 傅尧, 化学学报, 2018, 76, 956.)
doi: 10.6023/A18080333 |
|
| [2] |
(a) O’Hagan, D.; Deng, H. Chem. Rev. 2015, 115, 634.
doi: 10.1021/cr500209t |
|
(b) Shi, Y.; Xiao, T.; Xia, D.; Yang, W. Chin. J. Org. Chem. 2022, 42, 2715. (in Chinese)
doi: 10.6023/cjoc202203041 |
|
|
(石云, 肖婷, 夏冬, 杨文超, 有机化学, 2022, 42, 2715.)
doi: 10.6023/cjoc202203041 |
|
|
(c) Chen, D.; Jiang, J.; Wan, J.-P. Chin. J. Chem. 2022, 40, 2582.
doi: 10.1002/cjoc.v40.21 |
|
| [3] |
(a) Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis Reactivity Applications, Wiley-VCH, Weinheim, Germany, 2007.
|
|
(b) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
doi: 10.1126/science.1131943 |
|
|
(c) Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214.
doi: 10.1002/anie.v52.32 |
|
| [4] |
(a) Lin, G.-Q.; You, Q.-D.; Cheng, J.-F. Chiral Drugs: Chemistry and Biological Action, John Wiley & Sons, Inc., Hoboken, 2011.
|
|
(b) Oishi, S.; Kamitani, H.; Kodera, Y.; Watanabe, K.; Kobayashi, K.; Narumi, T.; Tomita, K.; Ohno, H.; Naito, T.; Kodama, E.; Matsuoka, M.; Fujii, N. Org. Biomol. Chem. 2009, 7, 2872.
doi: 10.1039/b907983a |
|
|
(c) Meanwell, N. A. J. Med. Chem. 2018, 61, 5822.
doi: 10.1021/acs.jmedchem.7b01788 |
|
| [5] |
(a) Reddy, V. P. Organofluorine Compounds in Biology and Medicine, Elsevier, Amsterdam, 2015.
|
|
(b) Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, WileyVCH, Weinheim, 2013.
|
|
|
(c) Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496
doi: 10.1039/c0cs00221f |
|
| [6] |
(a) Drouin, M.; Paquin, J.-F. Beilstein. J. Org. Chem. 2017, 13, 2637.
doi: 10.3762/bjoc.13.262 pmid: 21399789 |
|
(b) Landelle, G.; Bergeron, M.; Turcotte-Savard, M.-O.; Paquin, J.-F. Chem. Soc. Rev. 2011, 40, 2867.
doi: 10.1039/c0cs00201a pmid: 21399789 |
|
|
(c) Drouin, M.; Hamel, J.-D.; Paquin, J.-F. Synthesis 2018, 50, 881.
doi: 10.1055/s-0036-1591867 pmid: 21399789 |
|
| [7] |
(a) Xu, J.; Ahmed, E.-A.; Xiao, B.; Lu, Q.-Q.; Wang, Y.-L.; Yu, C.-G.; Fu, Y. Angew. Chem., Int. Ed. 2015, 54, 8231.
doi: 10.1002/anie.201502308 |
|
(b) Jiang, Z.-T.; Huang, J.; Zeng, Y.; Hu, F.; Xia, Y. Angew. Chem., nt. Ed. 2021, 60, 10626.
|
|
| [8] |
(a) Wang, C.; Liu, Y.-C.; Xu, M.-Y.; Xiao, B. Org. Lett. 2021, 23, 4593.
doi: 10.1021/acs.orglett.1c01289 |
|
(b) Dutheuil, G.; Paturel, C.; Lei, X.; Couve-Bonnaire, S.; Pannecoucke, X. J. Org. Chem. 2006, 71, 4316.
doi: 10.1021/jo0604787 |
|
|
(c) Andrei, D.; Wnuk, S. F. J. Org. Chem. 2006, 71, 405.
doi: 10.1021/jo051980e |
|
|
(d) Schneider, C.; Masi, D.; Couve-Bonnaire, S.; Pannecoucke, X.; Hoarau, C. Angew. Chem.,Int. Ed. 2013, 52, 3246.
doi: 10.1002/anie.201209446 |
|
| [9] |
(a) Koley, S.; Altman, R. A. Isr. J. Chem. 2020, 60, 313.
doi: 10.1002/ijch.v60.3-4 |
|
(b) Ma, T.; Chen, Y.; Li, Y.; Ping, Y.; Kong, W. ACS Catal. 2019, 9, 9127.
doi: 10.1021/acscatal.9b03172 |
|
|
(c) Li, J.; Rao, W.; Wang, S.-Y.; Ji, S.-J. J. Org. Chem. 2019, 84, 11542.
doi: 10.1021/acs.joc.9b01387 |
|
|
(d) Yang, L.; Ji, W.-W.; Lin, E.; Li, J.-L.; Fan, W.-X.; Li, Q.; Wang, H. Org. Lett. 2018, 20, 1924.
doi: 10.1021/acs.orglett.8b00471 |
|
| [10] |
(a) Tian, P.; Feng, C.; Loh, T.-P. Nat. Commun. 2015, 6, 7472.
doi: 10.1038/ncomms8472 |
|
(b) Kong, L.; Zhou, X.; Li, X. Org. Lett. 2016, 18, 6320.
doi: 10.1021/acs.orglett.6b03203 |
|
|
(c) Zell, D.; Dhawa, U.; Müller, V.; Bursch, M.; Grimme, S.; Ackermann, L. ACS Catal. 2017, 7, 4209.
doi: 10.1021/acscatal.7b01208 |
|
|
(d) Fuchibe, K.; Mayumi, Y.; Zhao, N.; Watanabe, S.; Yokota, M.; Ichikawa, J. Angew. Chem., Int. Ed. 2013, 52, 7825.
doi: 10.1002/anie.v52.30 |
|
| [11] |
(a) Thornbury, R. T.; Toste, F. D. Angew. Chem., Int. Ed. 2016, 55, 11629.
doi: 10.1002/anie.201605651 pmid: 26077810 |
|
(b) Xiong, Y.; Huang, T.; Ji, X.; Wu, J.; Cao, S. Org. Biomol. Chem. 2015, 13, 7389.
doi: 10.1039/c5ob01016k pmid: 26077810 |
|
| [12] |
(a) Lu, X.; Wang, Y.; Zhang, B.; Pi, J.-J.; Wang, X.-X.; Gong, T.-J.; Xiao, B.; Fu, Y. J. Am. Chem. Soc. 2017, 139, 12632.
pmid: 30774912 |
|
(b) Du, H.-W.; Sun, J.; Gao, Q.-S.; Wang, J.-Y.; Wang, H.; Xu, Z.; Zhou, M.-D. Org. Lett. 2020, 22, 1542.
doi: 10.1021/acs.orglett.0c00134 pmid: 30774912 |
|
|
(c) Dai, W.; Shi, H.; Zhao, X.; Cao, S. Org. Lett. 2016, 18, 4284.
doi: 10.1021/acs.orglett.6b02026 pmid: 30774912 |
|
|
(d) Zhou, L.; Zhu, C.; Bi, P.; Feng, C. Chem. Sci. 2019, 10, 1144.
doi: 10.1039/c8sc04162h pmid: 30774912 |
|
|
(e) Xie, J.; Yu, J.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2016, 55, 9416.
doi: 10.1002/anie.v55.32 pmid: 30774912 |
|
|
(f) Yu, L.; Tang, M.-L.; Si, C.-M.; Meng, Z.; Liang, Y.; Han, J.; Sun, X. Org. Lett. 2018, 20, 4579.
doi: 10.1021/acs.orglett.8b01866 pmid: 30774912 |
|
|
(g) Yang, H.; Tian, C.; Qiu, D.; Tian, H.; An, G.; Li, G. Org. Chem. Front. 2019, 6, 2365.
doi: 10.1039/c9qo00495e pmid: 30774912 |
|
|
(h) Li, J.; Lefebvre, Q.; Yang, H.; Zhao, Y.; Fu, H. Chem. Commun. 2017, 53, 10299.
doi: 10.1039/C7CC05758J pmid: 30774912 |
|
| [13] |
Lu, X.-Y.; Gao, A.; Ge, M.-Y.; Xia, Z.-J.; Liu, Q.-L.; Tao, T.-H.; Sun, X.-M. J. Org. Chem. 2022, 87, 4654.
doi: 10.1021/acs.joc.1c03088 |
| [14] |
Lu, X.-Y.; Ge, M.-Y.; Tao, T.-H.; Sun, X.-M.; Gao, M.-T.; Bao, S.-T.; Liu, Q.-L.; Xia, Z.-J.; Xia, J. Org. Chem. Front. 2022, 9, 831.
doi: 10.1039/D1QO01567B |
| [15] |
Lu, X.-Y.; Chen, X.-K.; Gao, M.-T.; Sun, X.-M.; Jiang, R.-C.; Wang, J.-C.; Yu, L.-J.; Ge, M.-Y.; Wei, Z.-H.; Liu, Z. Org. Chem. Front. 2022, 9, 4712.
doi: 10.1039/D2QO00977C |
| [16] |
Chen, Y.; Du, J.; Zuo, Z. Chem 2020, 6, 266.
doi: 10.1016/j.chempr.2019.11.009 |
| [17] |
(a) Xiao, T.; Zhou, L.; Huang, H.; Anand, D. Synthesis 2020, 52, 1585.
doi: 10.1055/s-0039-1690844 |
|
(b) Yu, X.-Y.; Zhao, Q.-Q.; Chen, J.; Xiao, W.-J.; Chen, J.-R. Acc. Chem. Res. 2020, 53, 1066.
doi: 10.1021/acs.accounts.0c00090 |
|
|
(c) Xiao, F.; Guo, Y.; Zeng, Y. F. Adv. Synth. Catal. 2021, 363, 120.
doi: 10.1002/adsc.v363.1 |
|
|
(d) Sivaguru, P.; Wang, Z.; Zanoni, G.; Bi, X. Chem. Soc. Rev. 2019, 48, 2615.
doi: 10.1039/C8CS00386F |
|
|
(e) Xiao, W.; Wu, J. Chin. Chem. Lett. 2020, 31, 3083.
doi: 10.1016/j.cclet.2020.07.035 |
|
|
(f) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506.
doi: 10.1021/acs.chemrev.0c00030 |
|
|
(g) Lu, X.-Y.; Liu, C.-C.; Jiang, R.-C.; Yan, L.-Y.; liu, Q.-L.; Wang, Q.-Q.; Li, J.-M. Chem. Commun. 2020, 56, 14191.
doi: 10.1039/D0CC06517J |
|
|
(h) Lu, X.-Y.; Xia, Z.-J.; Gao, A.; Liu, Q.-L.; Jiang, R.-C.; Liu, C.-C. J. Org. Chem. 2021, 86, 8829.
doi: 10.1021/acs.joc.1c00726 |
|
| [18] |
(a) Nguyen, B.-N.; Cao, H.-T. Eur. J. Org. Chem. 2019, 20196, 5912.
|
|
(b) Matsumoto, A.; Nguyen, B.-N.; Honda, T.; Sakamoto, R.; Huang, X.; Sakaki, S.; Maruoka, K. Chem. Asian J. 2021, 16, 282.
doi: 10.1002/asia.v16.4 |
|
| [19] |
(a) Chen, L.; Zhang, L.; Yan, G.; Huang, D. Asian J. Org. Chem. 2020, 9, 842.
doi: 10.1002/ajoc.v9.6 |
|
(b) Lu, X.-Y.; Li, J.-S.; Wang, S.-Q.; Zhu, Y.-J.; Li, Y.-M.; Yan, L.-Y.; Li, J.-M.; Wang, J.-Y.; Zhou, H.-P.; Ge, X.-T. Chem. Commun. 2019, 55, 11123.
doi: 10.1039/C9CC04795F |
| [1] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [2] | 宋晓, 卿晶, 黎君, 贾雪雷, 吴福松, 黄均荣, 金剑, 游恒志. 铜催化格氏试剂的不对称烯丙基烷基化连续流反应[J]. 有机化学, 2023, 43(9): 3174-3179. |
| [3] | 王熠, 张键, 刘飏子, 罗晓燕, 邓卫平. 钯催化不对称[3+4]环加成构建吲哚并环庚烷[J]. 有机化学, 2023, 43(8): 2864-2877. |
| [4] | 鲍志成, 李慕尧, 王剑波. 铜催化芳基重氮乙酸酯与双[(频哪醇)硼基]甲烷的偶联反应[J]. 有机化学, 2023, 43(5): 1808-1814. |
| [5] | 李春生, 连晓琪, 陈莲芬. 铜催化亚砜叶立德与邻苯二胺[4+2]环加成反应[J]. 有机化学, 2023, 43(4): 1492-1498. |
| [6] | 刘洋, 黄翔, 王敏, 廖建. 铜催化环酮亚胺与β,γ-不饱和N-酰基吡唑不对称Mannich-Type反应[J]. 有机化学, 2023, 43(4): 1499-1509. |
| [7] | 梁志鹏, 叶浩, 张海滨, 姜国民, 吴新星. 环丁酮类腙参与的偕二氟环丙烷开环胺化反应[J]. 有机化学, 2023, 43(4): 1483-1491. |
| [8] | 刘春阳, 李燕, 张前. 铜催化环状烯烃烯丙位C(sp3)—H磺酰化反应研究[J]. 有机化学, 2023, 43(3): 1091-1101. |
| [9] | 韩彪, 李维双, 陈舒晗, 张泽浪, 赵雪, 张瑶瑶, 朱磊. 铜催化不饱和化合物硅加成反应的研究进展[J]. 有机化学, 2023, 43(2): 555-572. |
| [10] | 王永玲, 张铁欣, 张栩铭, 孙晗扬, 冷津瑶, 李亚明. 可见光催化N-芳基乙醛酸亚胺脱羧烷基化合成非天然氨基酸衍生物[J]. 有机化学, 2023, 43(12): 4284-4293. |
| [11] | 陈志远, 杨梦维, 徐建林, 徐允河. 铜催化双炔膦氧化物硅质子化反应合成β-硅基取代的乙烯基膦氧化物[J]. 有机化学, 2023, 43(10): 3598-3607. |
| [12] | 许力, 吕兰兰, 王香善. 铜催化烯醇硅醚与芳基亚磺酸钠合成β-酮砜的研究[J]. 有机化学, 2023, 43(10): 3644-3651. |
| [13] | 陈飞, 陶晟, 刘宁, 代斌. CNN型双核Cu(I)配合物室温催化固定CO2的直接羧基化反应[J]. 有机化学, 2022, 42(8): 2471-2480. |
| [14] | 李晖, 殷亮. 铜催化的直接型插烯反应研究进展[J]. 有机化学, 2022, 42(6): 1573-1585. |
| [15] | 孙天义, 张依凡, 孟远倢, 王怡, 朱琦峰, 姜玉新, 刘石惠. 可见光-铜共催化的糖类区域选择性氧烷基化反应[J]. 有机化学, 2022, 42(5): 1414-1422. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||