有机化学 ›› 2024, Vol. 44 ›› Issue (4): 1069-1093.DOI: 10.6023/cjoc202308024 上一篇 下一篇
所属专题: 有机光催化合辑
综述与进展
沈都益a,b,*( ), 李玲慧a, 靳鸽a, 梁雨佳a, 张欣慧a, 公培伟a, 张范军a,*(
), 李玲慧a, 靳鸽a, 梁雨佳a, 张欣慧a, 公培伟a, 张范军a,*( ), 晁绵冉a,*(
), 晁绵冉a,*( )
)
收稿日期:2023-08-27
修回日期:2023-11-03
发布日期:2023-11-15
基金资助:
Duyi Shena,b( ), Linghui Lia, Ge Jinga, Yujia Lianga, Xinhui Zhanga, Peiwei Gonga, Fanjun Zhanga(
), Linghui Lia, Ge Jinga, Yujia Lianga, Xinhui Zhanga, Peiwei Gonga, Fanjun Zhanga( ), Mianran Chaoa(
), Mianran Chaoa( )
)
Received:2023-08-27
Revised:2023-11-03
Published:2023-11-15
Contact:
E-mail: Supported by:文章分享

自然界的黄素辅酶具有结构简单、无毒、能吸收和利用可见光等特点, 常常作为电子载体参与生物体内的氧化还原反应. 受黄素催化氧化功能的启发, 过去二十年里, 黄素衍生物催化以氧气或双氧水为终端氧化剂的两电子氧化反应得到了长足发展; 而近年来, 由于黄素衍生物丰富易调控的光化学性质, 更多的注意力开始转向它们所促进的单电子转移反应, 相应的有机合成方法学也正在涌现. 综述了截止到2023年7月的可见光驱动黄素衍生物催化诸如芳香环、含氮、含氧及含硫等官能团的单电子氧化, 生成相应的阳离子自由基中间体再参与后续过程来实现多样化有机转化的相关进展. 同时, 对底物范围和单电子转移机理进行了讨论, 并对该领域的未来发展进行了展望.
沈都益, 李玲慧, 靳鸽, 梁雨佳, 张欣慧, 公培伟, 张范军, 晁绵冉. 基于单电子转移的黄素仿生光催化氧化研究进展[J]. 有机化学, 2024, 44(4): 1069-1093.
Duyi Shen, Linghui Li, Ge Jing, Yujia Liang, Xinhui Zhang, Peiwei Gong, Fanjun Zhang, Mianran Chao. Advances in Flavin-Inspired Photocatalytic Oxidations Involving Single Electron Transfer Process[J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1069-1093.
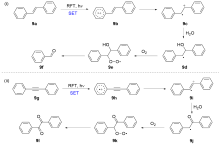
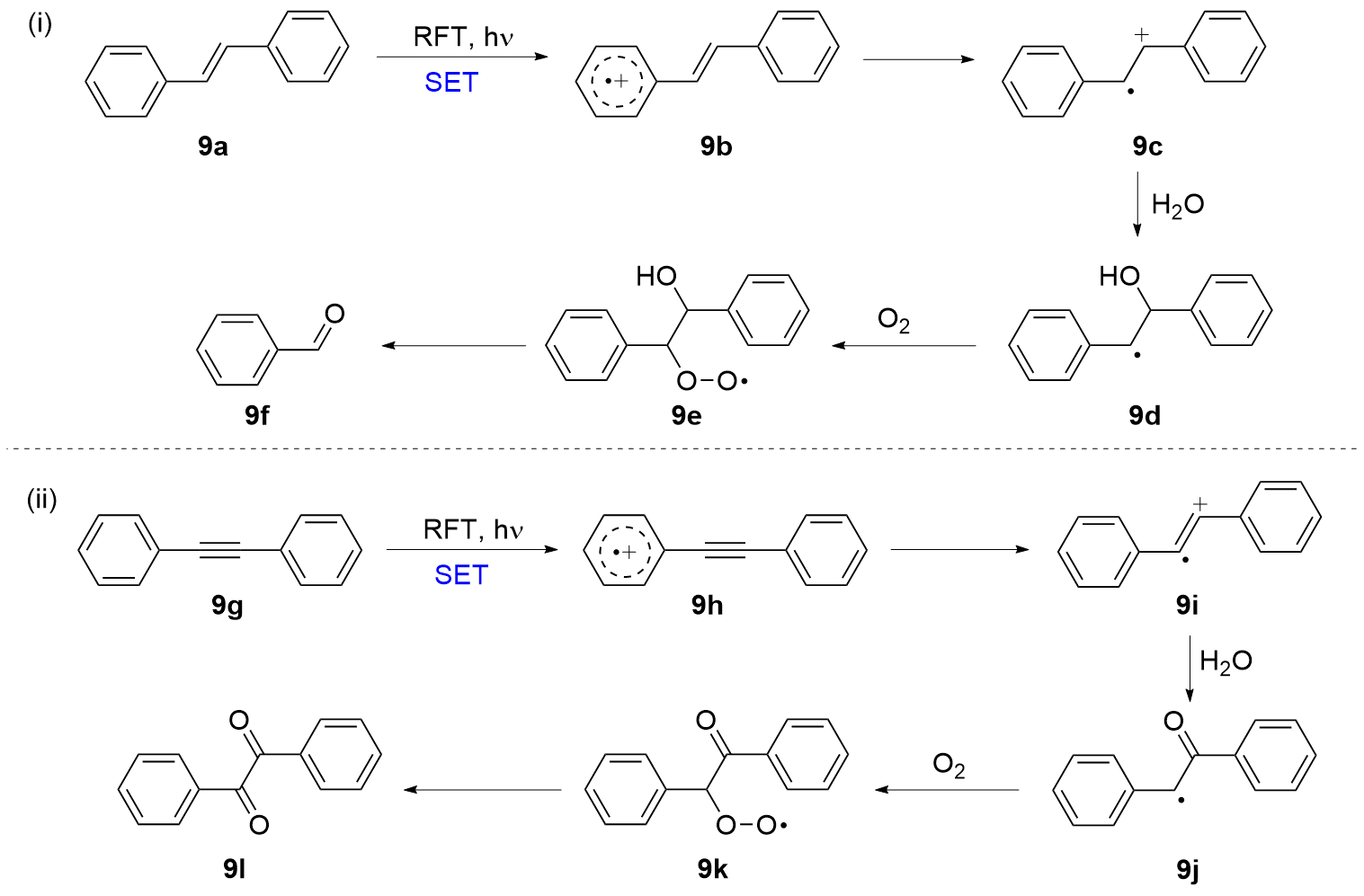
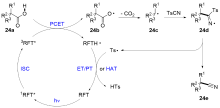
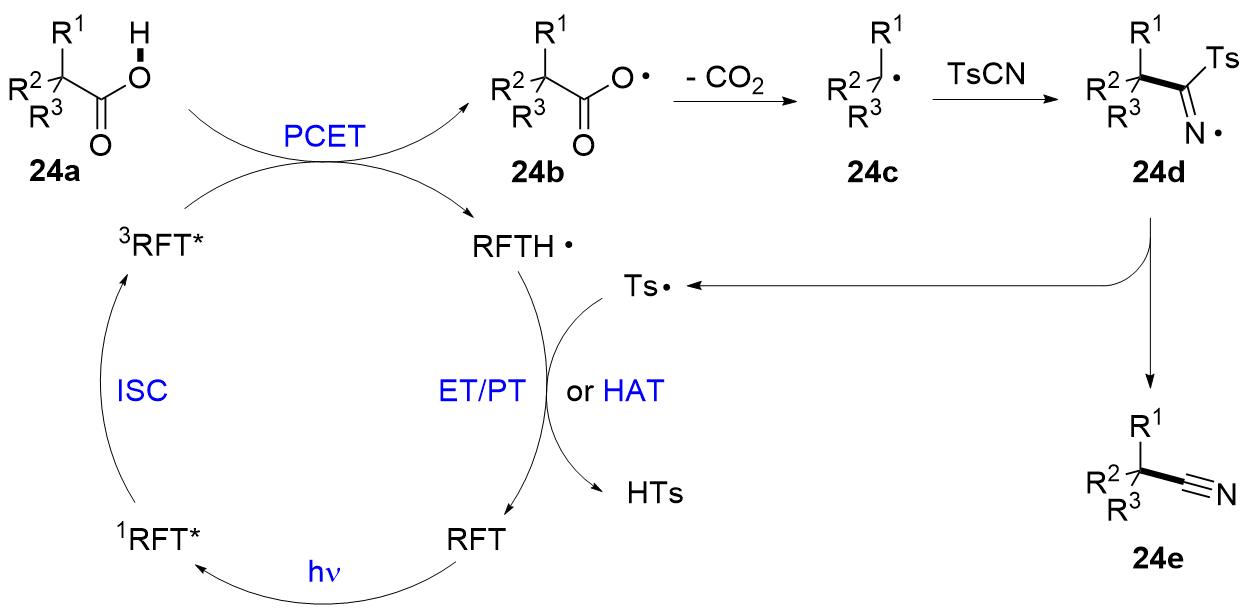
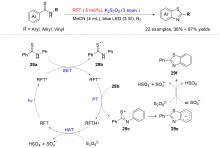
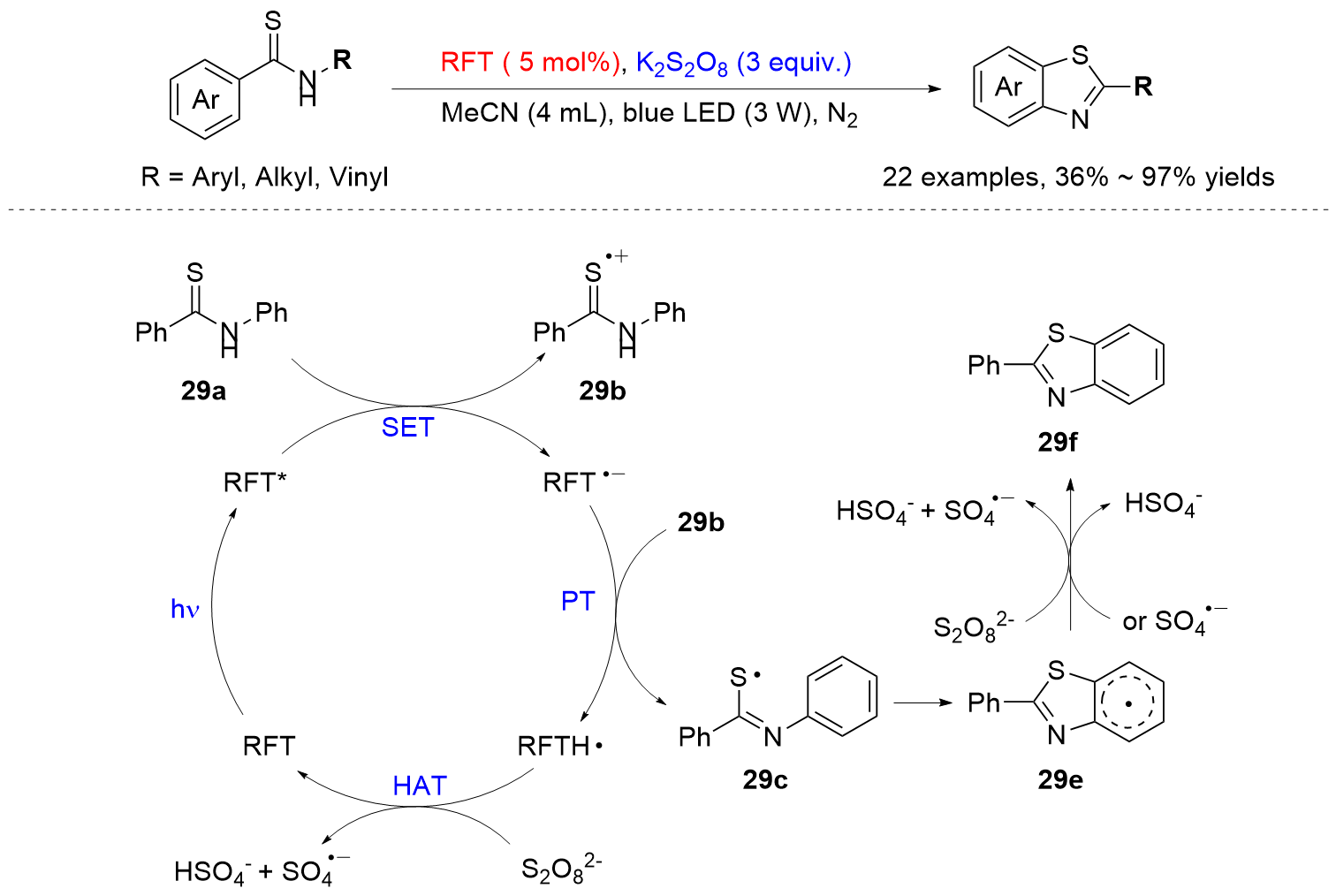
| [1] |
(a) Holmberg-Douglas N.; Nicewicz D. A. Chem. Rev. 2022, 122, 1925.
doi: 10.1021/acs.chemrev.1c00311 |
|
(b) Yu X.-Y.; Chen J.-R.; Xiao W.-J. Chem. Rev. 2021, 121, 506.
doi: 10.1021/acs.chemrev.0c00030 |
|
|
(c) Cheung K. P. S.; Sarkar S.; Gevorgyan V. Chem. Rev. 2022, 122, 1543.
doi: 10.1021/acs.chemrev.1c00403 |
|
|
(d) Teegardin K.; Day J. I.; Chan J.; Weaver J. Org. Process Res. Dev. 2016, 20, 1156.
doi: 10.1021/acs.oprd.6b00101 |
|
|
(e) Shaw M. H.; Twilton J.; MacMillan D. W. C. J. Org. Chem. 2016, 81, 6898.
doi: 10.1021/acs.joc.6b01449 |
|
| [2] |
(a) Srivastava V.; Singh P. K.; Tivaria S.; Singh P. P. Org. Chem. Front. 2022, 9, 1485.
doi: 10.1039/D1QO01602D |
|
(b) Pitre S. P.; Overman L. E. Chem. Rev. 2022, 122, 1717.
doi: 10.1021/acs.chemrev.1c00247 |
|
|
(c) Sun C.-L.; Shi Z.-J. Chem. Rev. 2014, 114, 9219.
doi: 10.1021/cr400274j |
|
| [3] |
(a) Romero N. A.; Nicewicz D. A. Chem. Rev. 2016, 116, 10075.
doi: 10.1021/acs.chemrev.6b00057 |
|
(b) Sideri I. K.; Voutyritsa E.; Kokotos C. G. Org. Biomol. Chem. 2018, 16, 4596.
doi: 10.1039/C8OB00725J |
|
|
(c) Fukuzumi S.; Ohkubo K. Org. Biomol. Chem. 2014, 12, 6059.
doi: 10.1039/C4OB00843J |
|
| [4] |
(a) Lechner H.; Oberdorfer G. ChemBioChem 2022, 23, e202100599.
doi: 10.1002/cbic.v23.13 |
|
(b) Liang Y.; Wei J.; Qiu X.; Jiao N. Chem. Rev. 2018, 118, 4912.
doi: 10.1021/acs.chemrev.7b00193 |
|
| [5] |
(a) Romero E.; Castellanos J. R. G.; Gadda G.; Fraaije M. W.; Mattevi A. Chem. Rev. 2018, 118, 1742.
doi: 10.1021/acs.chemrev.7b00650 |
|
(b) Silva E.; Edwards A. M. Flavins: Photochemistry and Photobiology, The Royal Society of Chemistry, Cambridge, 2002.
|
|
| [6] |
Ghisla S.; Kenney W. C.; Knappe W. R.; McIntire W.; Singer T. P. Biochemistry 1980, 19, 2537.
pmid: 6249335 |
| [7] |
(a) König B.; Kümmel S.; Svobodová E.; Cibulka R. Phys. Sci. Rev. 2018, 3, 20170168.
|
|
(b) Cibulka R.; Fraaije M. W. Flavin-Based Catalysis: Principles and Applications, Wiley-VCH GmbH, Weinheim, 2021.
|
|
| [8] |
Fukuzumi S.; Yasui K.; Suenobu T.; Ohkubo K.; Fujitsuka M.; Ito O. J. Phys. Chem. A 2001, 105, 10501.
doi: 10.1021/jp012709d |
| [9] |
Emmanuel M. A.; Bender S. G.; Bilodeau C.; Carceller J. M.; DeHovitz J. S.; Fu H.; Liu Y.; Nicholls B. T.; Ouyang Y.; Page C. G.; Qiao T.; Raps F. C.; Sorigué D. R.; Sun S.-Z.; Turek-Herman J.; Ye Y.; Rivas-Souchet A.; Cao J.; Hyster T. K. Chem. Rev. 2023, 123, 5459.
doi: 10.1021/acs.chemrev.2c00767 pmid: 37115521 |
| [10] |
(a) Litman Z. C.; Wang Y.; Zhao H.; Hartwig J. F. Nature 2018, 560, 355.
doi: 10.1038/s41586-018-0413-7 |
|
(b) Huang X.; Wang B.; Wang Y.; Jiang G.; Feng J.; Zhao H. Nature 2020, 584, 69.
doi: 10.1038/s41586-020-2406-6 |
|
|
(c) Harrison W.; Huang X.; Zhao H. Acc. Chem. Res. 2022, 55, 1087.
doi: 10.1021/acs.accounts.1c00719 |
|
| [11] |
(a) Oelgemöller M. Chem. Rev. 2016, 116, 9664.
doi: 10.1021/acs.chemrev.5b00720 |
|
(b) Ding W.; Lu L.‐Q.; Xiao W.‐J. In Green Oxidation in Organic Synthesis, 1st ed., Eds.: Jiao, N.; Stahl, S. S., John Wiley & Sons Ltd., Hoboken, NJ, 2019.
|
|
| [12] |
(a) Rehpenn A.; Walte A.; Storch G. Synthesis 2021, 53, 2583.
doi: 10.1055/a-1458-2419 pmid: 26077635 |
|
(b) de Gonzalo G.; Fraaije M. W. ChemCatChem 2013, 5, 403.
doi: 10.1002/cctc.v5.2 pmid: 26077635 |
|
|
(c) Iida H.; Imada Y.; Murahashi S.-I. Org. Biomol. Chem. 2015, 13, 7599.
doi: 10.1039/c5ob00854a pmid: 26077635 |
|
|
(d) Cibulka R. Eur. J. Org. Chem. 2015, 2015, 915.
doi: 10.1002/ejoc.v2015.5 pmid: 26077635 |
|
| [13] |
Srivastava V.; Singh P. K.; Srivastava A.; Singh P. P. RSC Adv. 2021, 11, 14251.
doi: 10.1039/D1RA00925G |
| [14] |
Fukuzumi S.; kuroda S.; Tanak T. Chem. Lett. 1984, 417.
|
| [15] |
Fukuzumi S.; Kuroda S.; Tanaka T. Chem. Lett. 1984, 1375.
|
| [16] |
Fukuzumi S.; Kuroda S.; Tanaka T. J. Am. Chem. Soc. 1985, 107, 3020.
doi: 10.1021/ja00297a005 |
| [17] |
(a) Fukuzumi S.; Kojima T. J. Biol. Inorg. Chem. 2008, 13, 321.
doi: 10.1007/s00775-008-0343-1 |
|
(b) Fukuzumi S.; Ohkubo K. Coord. Chem. Rev. 2010, 254, 372.
doi: 10.1016/j.ccr.2009.10.020 |
|
|
(c) Fukuzumi S.; Jung J.; Lee Y.-M.; Nam W. Asian J. Org. Chem. 2017, 6, 397.
doi: 10.1002/ajoc.v6.4 |
|
| [18] |
Mühldorf B.; Wolf R. Chem. Commun. 2015, 51, 8425.
doi: 10.1039/C5CC00178A |
| [19] |
Fukuzumi S.; Tanii K.; Tanaka T. J. Chem. Soc., Chem. Commun. 1989, 816.
|
| [20] |
Mühldorf B.; Wolf R. ChemCatChem 2017, 9, 920.
doi: 10.1002/cctc.v9.6 |
| [21] |
Zelenka J.; Svobodová E.; Tarábek J.; Hoskovcová I.; Bogus- chová V.; Bailly S.; Sikorski M.; Roithová J.; Cibulka R. Org. Lett. 2019, 21, 114.
doi: 10.1021/acs.orglett.8b03547 pmid: 30582822 |
| [22] |
Tolba A. H.; Vávra F.; Chudoba J.; Cibulka R. Eur. J. Org. Chem. 2020, 2020, 1579.
|
| [23] |
Pokluda A.; Anwar Z.; Boguschová V.; Anusiewicz I.; Skurski P.; Sikorski M.; Cibulka R. Adv. Synth. Catal. 2021, 363, 4371.
doi: 10.1002/adsc.v363.18 |
| [24] |
Graml A.; Neveselý T.; Kutta R. J.; Cibulka R.; König B. Nat. Commun. 2020, 11, 3174.
doi: 10.1038/s41467-020-16909-y |
| [25] |
Obertík R.; Chudoba J.; Šturala J.; Tarábek J.; Ludvíková L.; Slanina T.; König B.; Cibulka R. Chem.-Eur. J. 2022, 28, e202202487.
doi: 10.1002/chem.v28.67 |
| [26] |
Lechner R.; Kümmel S.; König B. Photochem. Photobiol. Sci. 2010, 9, 1367.
doi: 10.1039/c0pp00202j |
| [27] |
Taeufer T.; Cordero M. A. A.; Petrosyan A.; Surkus A.-E.; Lochbrunner S.; Pospech J. ChemPhotoChem 2021, 5, 999.
doi: 10.1002/cptc.v5.11 |
| [28] |
(a) El-Hage F.; Schöll C.; Pospech J. J. Org. Chem. 2020, 85, 13853.
doi: 10.1021/acs.joc.0c01955 |
|
(b) Taeufer T.; Hauptmann R.; El-Hage F.; Mayer T. S.; Jiao H.; Rabeah J.; Pospech J. ACS Catal. 2021, 11, 4862.
doi: 10.1021/acscatal.0c05540 |
|
| [29] |
Aleksander V. Mini-Rev. Org. Chem. 2017, 14, 204.
|
| [30] |
Shen D.; Zhong F.; Li L.; Zhang H.; Ren T.; Sun C.; Wang B.; Guo M.; Chao M.; Fukuzumi S. Org. Chem. Front. 2023, 10, 2653.
doi: 10.1039/D3QO00375B |
| [31] |
Düsel S. J. S.; König B. J. Org. Chem. 2018, 83, 2802.
doi: 10.1021/acs.joc.7b03260 |
| [32] |
Badenock J. C. Radical Reactions of Indole In Heterocyclic Scaffolds II: Topics in Heterocyclic Chemistry, Vol. 26, Ed.: Gribble, G., Springer, Berlin, 2010.
|
| [33] |
Immel J. R.; Alghafli B. M.; Ugalde A. A. R.; Bloom S. Org. Lett. 2023, 25, 3818.
doi: 10.1021/acs.orglett.3c01398 |
| [34] |
(a) Weinberg D. R.; Gagliardi C. J.; Hull J. F.; Murphy C. F.; Kent C. A.; Westlake B. C.; Paul A.; Ess D. H.; McCafferty D. G.; Meyer T. J. Chem. Rev. 2012, 112, 4016.
doi: 10.1021/cr200177j pmid: 33405896 |
|
(b) Tyburski R.; Liu T.; Glover S. D.; Hammarström L. J. Am. Chem. Soc. 2021, 143, 560.
doi: 10.1021/jacs.0c09106 pmid: 33405896 |
|
| [35] |
Adenier A.; Chehimi M. M.; Gallardo I.; Pinson J.; Vila N. Langmuir 2004, 20, 8243.
pmid: 15350099 |
| [36] |
Bertolotti S. G.; Previtali C. M.; Rufs A. M.; Encinas M. V. Macromolecules 1999, 32, 2920.
doi: 10.1021/ma981246f |
| [37] |
Porcal G.; Bertolotti S. G.; Previtali C. M.; Encinas M. V. Phys. Chem. Chem. Phys. 2003, 5, 4123.
doi: 10.1039/b306945a |
| [38] |
Martinez-Haya R.; Miranda M. A.; Marin M. L. Eur. J. Org. Chem. 2017, 2017, 2164.
|
| [39] |
Tagami T.; Arakawa Y.; Minagawa K.; Imada Y. Org. Lett. 2019, 21, 6978.
doi: 10.1021/acs.orglett.9b02567 |
| [40] |
Brown D. G.; Boström J. J. Med. Chem. 2016, 59, 4443.
doi: 10.1021/acs.jmedchem.5b01409 pmid: 26571338 |
| [41] |
Tolba A. H.; Krupicka M.; Chudoba J.; Cibulka R. Org. Lett. 2021, 23, 6825.
doi: 10.1021/acs.orglett.1c02391 |
| [42] |
Wu M.; Jiang Q.; Tian Q.; Guo T.; Cai F.; Tang S.; Liu J.; Wang X. CCS Chem. 2022, 4, 3599.
doi: 10.31635/ccschem.022.202101621 |
| [43] |
(a) Wu Y.; Zhou G.; Meng Q.; Tang X.; Liu G.; Yin H.; Zhao J.; Yang F.; Yu Z.; Luo Y. J. Org. Chem. 2018, 83, 13051.
doi: 10.1021/acs.joc.8b01710 |
|
(b) Luo K.; Yu X.; Chen P.; He K.; Lin J.; Jin Y. Tetrahedron Lett. 2020, 61, 151578.
doi: 10.1016/j.tetlet.2019.151578 |
|
|
(c) Zhang Y.; Yang X.; Tang H.; Liang D.; Wu J.; Huang D. Green Chem. 2020, 22, 22.
doi: 10.1039/C9GC03152A |
|
| [44] |
Shen D.; Ren T.; Luo Z.; Sun F.; Han Y.; Chen K.; Zhang X.; Zhou M.; Gong P.; Chao M. Org. Biomol. Chem. 2023, 21, 4955.
doi: 10.1039/D3OB00663H |
| [45] |
(a) Marin M. L.; Santos-Juanes L.; Arques A.; Amat A. M.; Miranda M. A. Chem. Rev. 2012, 112, 1710.
doi: 10.1021/cr2000543 |
|
(b) Pavanello A.; Miranda M. A.; Marin M. L. Chem. Eng. J. Adv. 2022, 11, 100296.
doi: 10.1016/j.ceja.2022.100296 |
|
| [46] |
(a) Krylov I. B.; Vil’ V. A.; Terent’ev A. O. Beilstein J. Org. Chem. 2015, 11, 92.
doi: 10.3762/bjoc.11.13 pmid: 25670997 |
|
(b) Li M.; Hong J.; Xiao W.; Yang Y.; Qiu D.; Mo F. ChemSusChem 2020, 13, 1661.
doi: 10.1002/cssc.v13.7 pmid: 25670997 |
|
| [47] |
Bloom S.; Liu C.; Kölmel D. K.; Qiao J. X.; Zhang Y.; Poss M. A.; Ewing W. R.; MacMillan D. W. C. Nat. Chem. 2018, 10, 205.
doi: 10.1038/nchem.2888 |
| [48] |
Sakakibara Y.; Murakami K. ACS Catal. 2022, 12, 1857.
doi: 10.1021/acscatal.1c05318 |
| [49] |
Morack T.; Metternich J. B.; Gilmour R. Org. Lett. 2018, 20, 1316.
doi: 10.1021/acs.orglett.8b00052 |
| [50] |
Ramirez N. P.; König B.; Gonzalez-Gomez J. C. Org. Lett. 2019, 21, 1368.
doi: 10.1021/acs.orglett.9b00064 pmid: 30785298 |
| [51] |
Ramirez N. P.; Lana-Villarreal T.; Gonzalez-Gomez J. C. Eur. J. Org. Chem. 2020, 2020, 1539.
doi: 10.1002/ejoc.201900888 |
| [52] |
Zhang B.; Wu H.; Li S.; Liu Y.; Du P.; Wang Z.-G. ACS Catal. 2023, 13, 6763.
doi: 10.1021/acscatal.3c01669 |
| [53] |
(a) Ji H.-F.; Shen L. J. Mol. Struct.: THEOCHEM 2008, 865, 25.
doi: 10.1016/j.theochem.2008.06.012 |
|
(b) Shen L.; Ji H.-F. J. Photochem. Photobiol. A 2008, 199, 119.
doi: 10.1016/j.jphotochem.2008.04.002 |
|
|
(c) Ji H.-F.; Shen L. J. Mol. Struct.: THEOCHEM 2008, 862, 148.
doi: 10.1016/j.theochem.2008.05.003 |
|
| [54] |
Roche S. P.; Porco J. A. Jr. Angew. Chem., Int. Ed. 2011, 50, 4068.
doi: 10.1002/anie.v50.18 |
| [55] |
Dockrey S. A. B.; Narayan A. R. H. Org. Lett. 2020, 22, 3712.
doi: 10.1021/acs.orglett.0c01207 pmid: 32293185 |
| [56] |
Skolia E.; Gkizis P. L.; Kokotos C. G. ChemPlusChem 2022, 87, e202200008.
doi: 10.1002/cplu.v87.4 |
| [57] |
(a) Dad’ová J.; Svobodová E.; Sikorski M.; König B.; Cibulka R. ChemCatChem 2012, 4, 620.
doi: 10.1002/cctc.v4.5 |
|
(b) Neveselý T.; Svobodová E.; Chudoba J.; Sikorski M.; Cibulka R. Adv. Synth. Catal. 2016, 358, 1654.
doi: 10.1002/adsc.v358.10 |
|
| [58] |
Bouchet L. M.; Heredia A. A.; Argüello J. E.; Schmidt L. C. Org. Lett. 2020, 22, 610.
doi: 10.1021/acs.orglett.9b04384 pmid: 31887062 |
| [59] |
Arakawa Y.; Mihara T.; Fujii H.; Minagawa K.; Imada Y. Chem. Commun. 2020, 56, 5661.
doi: 10.1039/D0CC01960G |
| [60] |
Kim Y.; Ho S. O.; Gassman N. R.; Korlann Y.; Landorf E. V.; Collart F. R.; Weiss S. Bioconjugate Chem. 2008, 19, 786.
doi: 10.1021/bc7002499 |
| [61] |
Kim J.; Li B. X.; Huang R. Y.-C.; Qiao J. X.; Ewing W. R.; MacMillan D. W. C. J. Am. Chem. Soc. 2020, 142, 21260.
doi: 10.1021/jacs.0c09926 |
| [62] |
(a) Fletcher S. Org. Chem. Front. 2015, 2, 739.
doi: 10.1039/C5QO00016E |
|
(b) Beddoe R. H.; Andrews K. G.; Magné V.; Cuthbertson J. D.; Saska J.; Shannon-Little A. L.; Shanahan S. E.; Sneddon H. F.; Denton R. M. Science 2019, 365, 910.
doi: 10.1126/science.aax3353 |
|
| [63] |
März M.; Kohout M.; Neveselý T.; Chudoba J.; Prukała D.; Niziński S.; Sikorski M.; Burdziński G.; Cibulka R. Org. Biomol. Chem. 2018, 16, 6809.
doi: 10.1039/C8OB01822G |
| [64] |
(a) Yan M.; Lo J. C.; Edwards J. T.; Baran P. S. J. Am. Chem. Soc. 2016, 138, 12692.
doi: 10.1021/jacs.6b08856 pmid: 30101272 |
|
(b) Romero K. J.; Galliher M. S.; Pratt D. A.; Stephenson C. R. J. Chem. Soc. Rev. 2018, 47, 7851.
doi: 10.1039/c8cs00379c pmid: 30101272 |
|
| [65] |
Chilamari M.; Immel J. R.; Bloom S. ACS Catal. 2020, 10, 12727.
doi: 10.1021/acscatal.0c03422 |
| [66] |
(a) Lu C.; Yao S.; Han Z.; Lin W.; Wang W.; Zhang W.; Lin N. Biophys. Chem. 2000, 85, 17.
pmid: 24769501 |
|
(b) Chen W.; Chen J.-J.; Lu R.; Qian C.; Li W.-W.; Yu H.-Q. Bioelectrochemistry 2014, 98, 103.
doi: 10.1016/j.bioelechem.2014.03.010 pmid: 24769501 |
|
| [67] |
Du P.; Shen Y.; Zhang B.; Li S.; Gao M.; Wang T.; Ding X.; Yu B.; Wang Z.-G.; Xu F.-J. Adv. Sci. 2023, 10, 2206851.
doi: 10.1002/advs.v10.9 |
| [1] | 鞠国栋, 周冠宇, 赵应声. 三异丙基硅烷(TIPS)保护苯酚的无过渡金属催化区域选择性硫氰化反应[J]. 有机化学, 2024, 44(4): 1327-1336. |
| [2] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [3] | 徐伟, 翟宏斌, 程斌, 汪太民. 可见光诱导的钯催化Heck反应[J]. 有机化学, 2023, 43(9): 3035-3054. |
| [4] | 杨晓娜, 郭宏宇, 周荣. 可见光促进有机硅化合物参与的化学转化[J]. 有机化学, 2023, 43(8): 2720-2742. |
| [5] | 陈祥, 欧阳文韬, 李潇, 何卫民. 可见光诱导有机光催化合成二氟乙基苯并噁嗪[J]. 有机化学, 2023, 43(12): 4213-4219. |
| [6] | 宋戈洋, 薛东. 光促进过渡金属催化的C-杂原子键偶联反应进展[J]. 有机化学, 2022, 42(8): 2275-2299. |
| [7] | 马丽文, 魏晓叶, 赵紫琳, 赵昂, 邓祥文, 霍丙南, 马刚, 张春芳. 端炔偶联反应中铜变价催化机制的理论研究[J]. 有机化学, 2022, 42(6): 1811-1819. |
| [8] | 余卫国, 王灵娜, 俞晓聪, 罗书平. 荧光染料和镍协同催化的脱羧羰基化反应[J]. 有机化学, 2022, 42(4): 1216-1223. |
| [9] | 史敦发, 王露, 夏春谷, 刘超. 可见光促进烷基硼化合物转化研究进展[J]. 有机化学, 2020, 40(11): 3605-3619. |
| [10] | 颜世强, 谢明现, 王玉杰, 李英霞. 灯盏花乙素半合成柳穿鱼叶苷研究[J]. 有机化学, 2019, 39(2): 412-418. |
| [11] | 陈锦杨, 李玉涵, 梅兰, 吴红谕. 新型光敏剂2, 4, 5, 6-四(9-咔唑基)间苯二腈在无金属有机光合成中的应用[J]. 有机化学, 2019, 39(11): 3040-3050. |
| [12] | 侯文涛, 贾晓东. 自由基正离子盐促进的化学转化研究进展[J]. 有机化学, 2018, 38(5): 999-1008. |
| [13] | 孔黎春, 周雨露, 罗芳, 朱钢国. 醛基为受体的氧化自由基加成反应研究进展[J]. 有机化学, 2018, 38(11): 2858-2865. |
| [14] | 阮利衡, 董振诚, 陈春欣, 吴爽, 孙京. 过渡金属镍与可见光双催化体系的研究进展[J]. 有机化学, 2017, 37(10): 2544-2554. |
| [15] | 杨永青, 朱孟楠, 陆征, 陈立婷, 苏静. 涉及碳-碳键断裂的克莱森-施密特缩合反应[J]. 有机化学, 2016, 36(5): 1073-1079. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||