有机化学 ›› 2024, Vol. 44 ›› Issue (11): 3518-3525.DOI: 10.6023/cjoc202405018 上一篇 下一篇
研究论文
收稿日期:2024-05-14
发布日期:2024-05-30
基金资助:Received:2024-05-14
Published:2024-05-30
Contact:
*E-mail:Supported by:文章分享

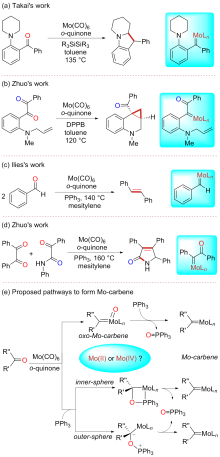
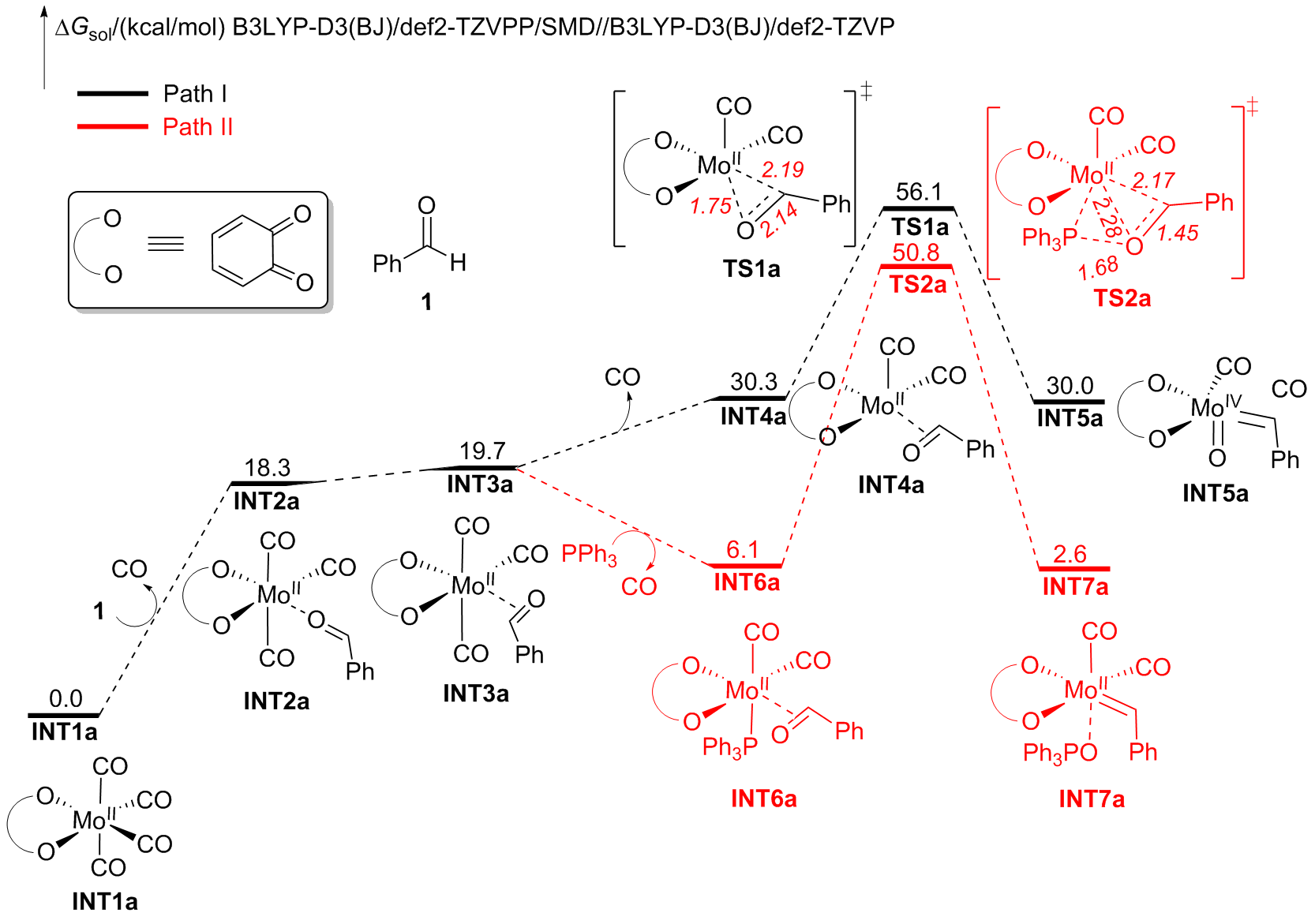
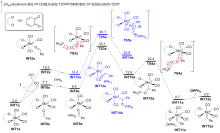
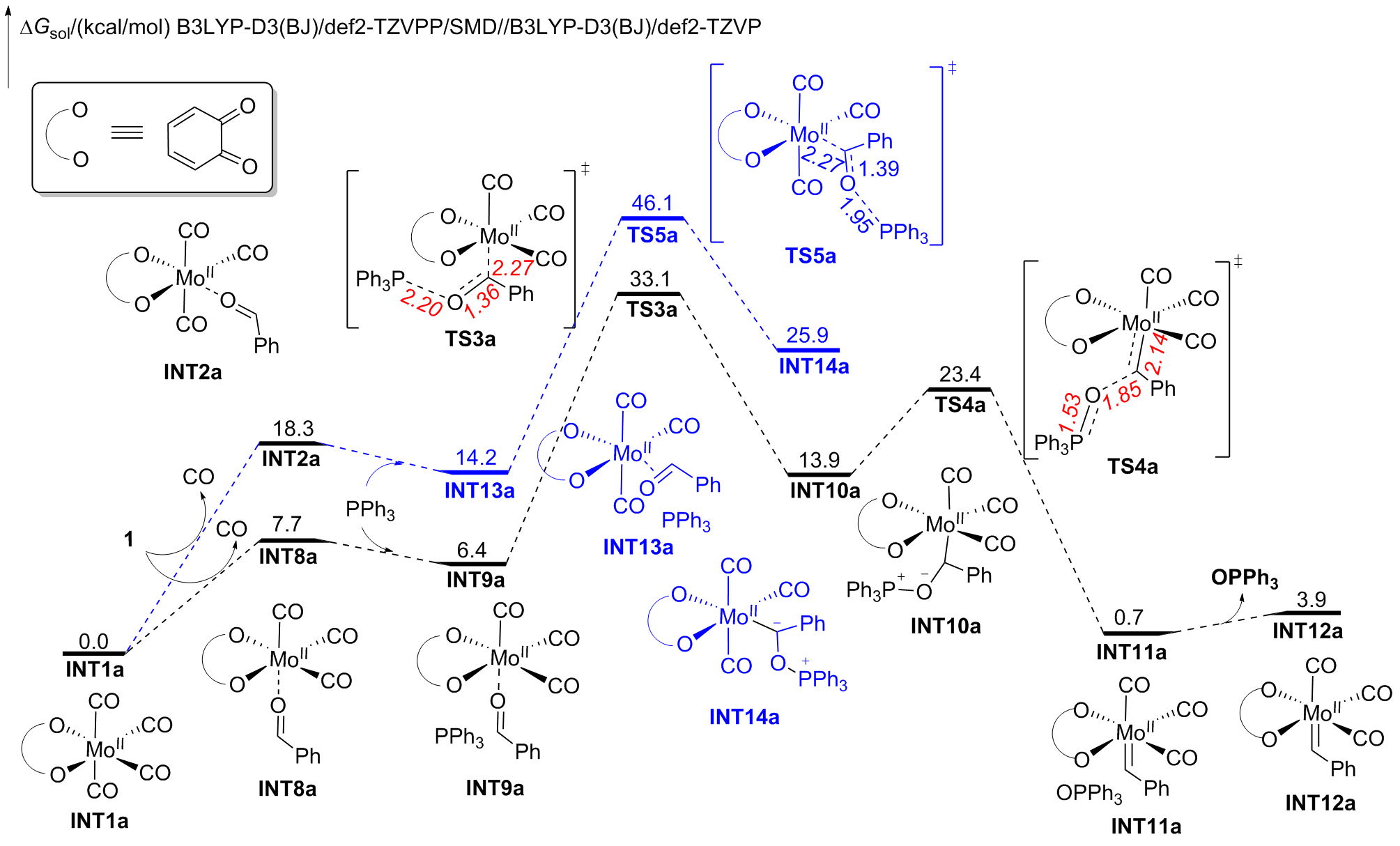
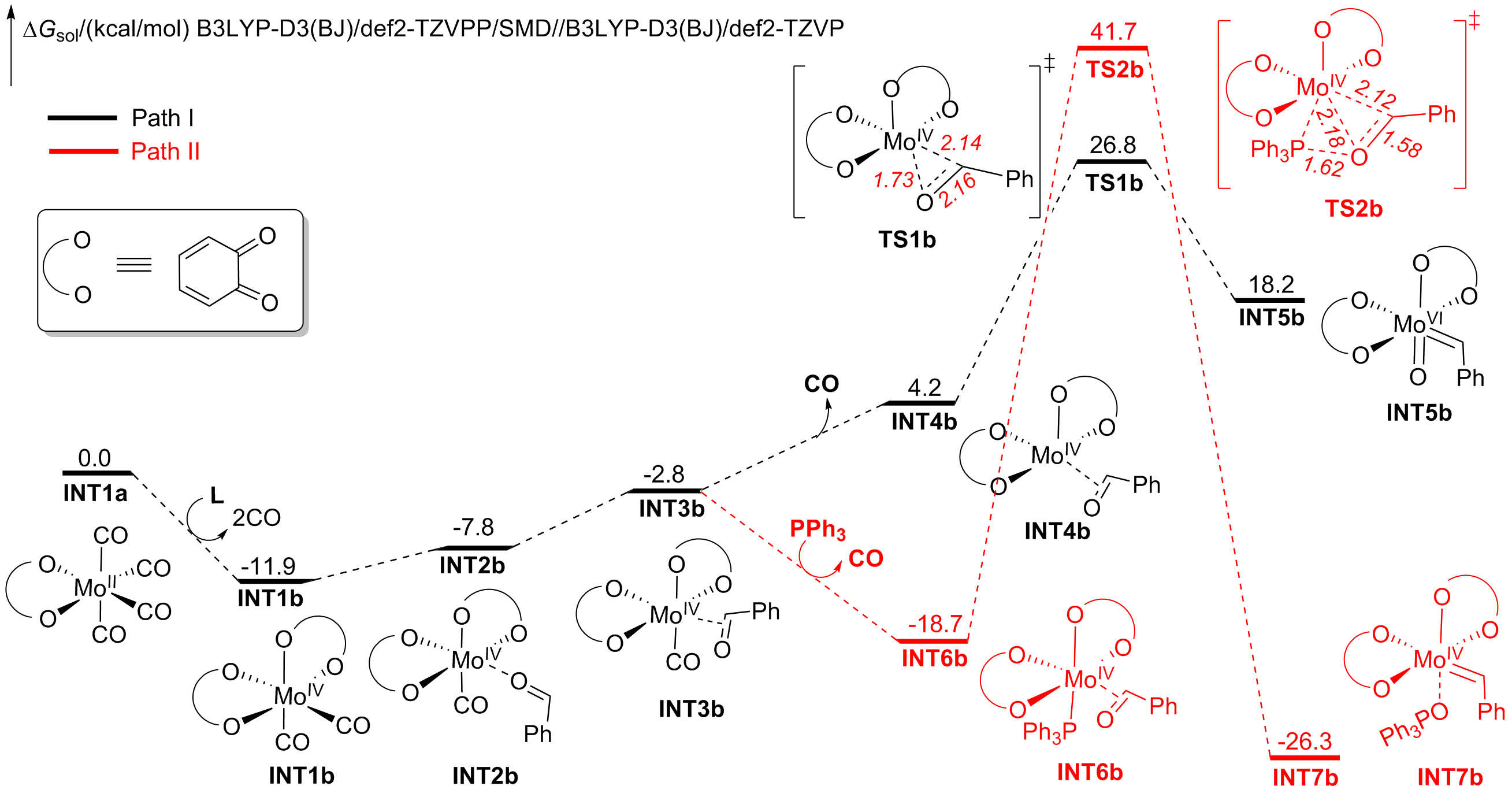
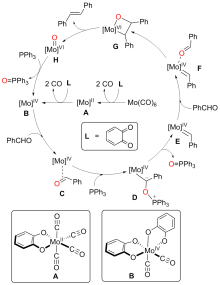
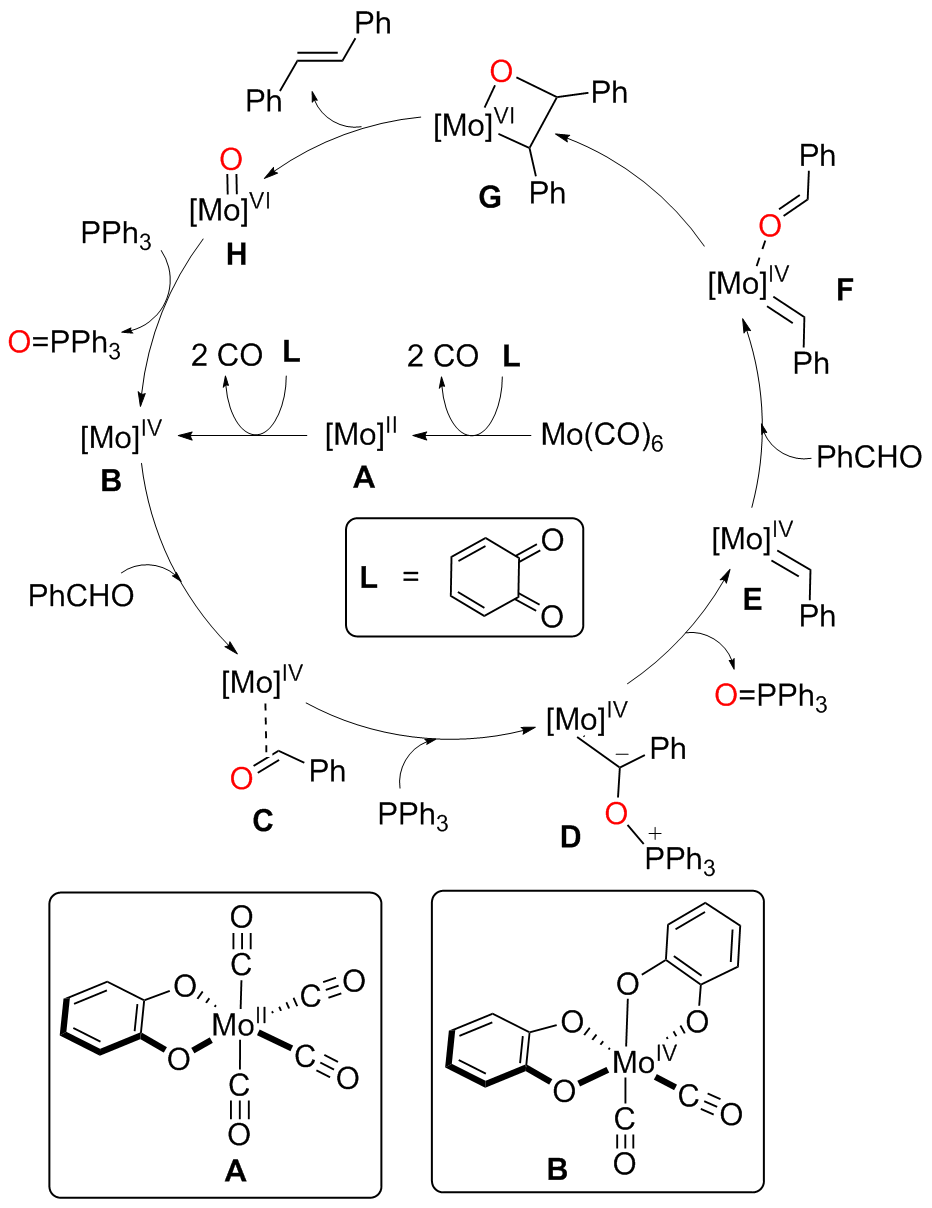
钼/邻醌配合物在适当还原剂存在下可以促进羰基脱氧并生成关键的钼卡宾中间体, 进而发生后续转化. 本文对钼催化芳香醛脱氧偶联反应机制进行了详细的理论研究. 计算结果表明, Mo(IV)配合物(含两个邻醌配体)比文献提出的Mo(II)配合物(含一个邻醌配体)更有利于促进芳香醛脱氧生成钼卡宾中间体, 提出了一种新颖的外球分步脱氧机制: 还原剂三苯基膦(PPh3)与醛基氧结合生成P—O键, 随后醛基部分C—O键断裂生成关键的Mo(IV)卡宾中间体. 而文献中普遍认为的羰基直接在Mo(II)上发生氧化加成得到氧钼卡宾中间体并非有利路径. 生成Schrock型Mo(IV)卡宾后, 底物芳香醛可以与该中间体发生[2+2]环加成, 进而通过复分解模式得到最终产物. 此外, 讨论了形成Mo(IV)卡宾的有利因素和烯烃产物顺反构型选择性产生的根源.
孙庆浩, 鲍晓光. 钼催化芳香醛脱氧偶联反应机制的理论研究[J]. 有机化学, 2024, 44(11): 3518-3525.
Qinghao Sun, Xiaoguang Bao. Computational Insights into the Mechanism of the Mo-Catalyzed Deoxygenative Coupling of Aromatic Aldehydes[J]. Chinese Journal of Organic Chemistry, 2024, 44(11): 3518-3525.
| [1] |
(a) Skell P. S.; Woodworth R. C. J. Am. Chem. Soc. 1956, 78, 4496.
pmid: 37123600 |
|
(b) Doyle M. P. Chem. Rev. 1986, 86, 919.
pmid: 37123600 |
|
|
(c) Xia Y.; Qiu D.; Wang J. Chem. Rev. 2017, 117, 13810.
pmid: 37123600 |
|
|
(d) Bergstrom B. D.; Nickerson L. A.; Shaw J. T.; Souza L. W. Angew. Chem., Int. Ed. 2021, 60, 6864.
pmid: 37123600 |
|
|
(e) Epping R. F. J.; Vesseur D.; Zhou M.; De Bruin B. ACS Catal. 2023, 13, 5428.
doi: 10.1021/acscatal.3c00591 pmid: 37123600 |
|
| [2] |
For metal-carbene involving C—H insertions, see: (a) Choi M. K. W.; Yu W. Y.; Che C. M. Org. Lett. 2005, 7, 1081.
|
|
(b) Doyle M. P.; Duffy R.; Ratnikov M.; Zhou L. Chem. Rev. 2010, 110, 704.
|
|
|
(c) Lo V. K. Y.; Guo Z.; Choi M. K. W.; Yu W. Y.; Huang J. S.; Che C.-M. J. Am. Chem. Soc. 2012, 134, 7588.
|
|
|
(d) Shen H.; Xiao X.; Haj M. K.; Willoughby P. H.; Hoye T. R. J. Am. Chem. Soc. 2018, 140, 15616.
|
|
|
(e) He Y.; Huang Z.; Wu K.; Ma J.; Zhou Y.-G.; Yu Z. Chem. Soc. Rev. 2022, 51, 2759.
|
|
| [3] |
For metal-carbene involving X—H insertions, see: (a) Gillingham D.; Fei N. Chem. Soc. Rev. 2013, 42, 4918.
doi: 10.1039/c3cs35496b pmid: 35748338 |
|
(b) Yang Z.; Stivanin M. L.; Jurberg I. D.; Koenigs R. M. Chem. Soc. Rev. 2020, 49, 6833.
pmid: 35748338 |
|
|
(c) Chen P.; Nan J.; Hu Y.; Kang Y.; Wang B.; Ma Y.; Szostak M. Chem. Sci. 2021, 12, 803.
pmid: 35748338 |
|
|
(d) Roose T. R.; Verdoorn D. S.; Mampuys P.; Ruijter E.; Maes B. U. W.; Orru R. V. A. Chem. Soc. Rev. 2022, 51, 5842.
doi: 10.1039/d1cs00305d pmid: 35748338 |
|
| [4] |
For metal-carbene involving olefin metathesis, see: (a) Schrock R. R.; Hoveyda A. H. Angew. Chem., Int. Ed. 2003, 42, 4592.
pmid: 29714397 |
|
(b) Grela K. Olefin Metathesis—Theory and Practice, Wiley, Hoboken, NJ, 2014.
pmid: 29714397 |
|
|
(c) Ogba O. M.; Warner N. C.; O'Leary D. J.; Grubbs R. H. Chem. Soc. Rev. 2018, 47, 4510.
doi: 10.1039/c8cs00027a pmid: 29714397 |
|
|
(d) Goudreault A. Y.; Walden D. M.; Nascimento D. L.; Botti A. G.; Steinmann S. N.; Michel C.; Fogg D. E. ACS Catal. 2020, 10, 3838.
pmid: 29714397 |
|
| [5] |
For metal-carbene involving cyclization, see: (a) Dai X.-J.; Li C.-C.; Li C.-J. Chem. Soc. Rev. 2021, 50, 10733.
|
|
(b) Zhang Y.-H.; Shi B.-F.; Yu J.-Q. J. Am. Chem. Soc. 2009, 131, 5072.
|
|
| [6] |
Wang T.; Hashmi A. S. K. Chem. Rev. 2021, 121, 8948.
doi: 10.1021/acs.chemrev.0c00811 pmid: 33026800 |
| [7] |
(a) Feliciano A.; Vázquez J. L.; Benítez-Puebla L. J.; Velazco- Cabral I.; Cruz D.; Delgado F.; Vázquez M. A. Chem.-Eur. J. 2021, 27, 8233.
pmid: 15137791 |
|
(b) Barluenga J.; Santamaría J.; Tomás M. Chem. Rev. 2004, 104, 2259.
pmid: 15137791 |
|
|
(c) Schrock R. R. Chem. Rev. 2002, 102, 145.
pmid: 15137791 |
|
|
(d) Schrock R. R. Chem. Rev. 2009, 109, 3211.
pmid: 15137791 |
|
|
(e) Frenking G.; Solà M.; Vyboishchikov S. F. J. Organomet. Chem. 2005, 690, 6178.
pmid: 15137791 |
|
| [8] |
Harvey D.; Brown M. J. Am. Chem. Soc. 1990, 112, 7806.
|
| [9] |
Schrock R. R.; Murdzek J. S.; Bazan G. C.; Robbins J.; DiMare M.; O'Regan M. J. Am. Chem. Soc. 1990, 112, 3875.
|
| [10] |
(a) Asako S.; Ishihara S.; Hirata K.; Takai K. J. Am. Chem. Soc. 2019, 141, 9832.
doi: 10.1021/jacs.9b05428 pmid: 31184481 |
|
(b) Banerjee S.; Kobayashi T.; Takai K.; Asako S.; Ilies L. Org. Lett. 2022, 24, 7242.
pmid: 31184481 |
|
|
(c) Asako S.; Kobayashi T.; Ishihara S.; Takai K. Asian J. Org. Chem. 2021, 10, 753.
doi: 10.1002/ajoc.202100038 pmid: 31184481 |
|
| [11] |
(a) Cao L.-Y.; Luo J.-N.; Yao J.-S.; Wang D.-K.; Dong Y.-Q.; Zheng C.; Zhuo C.-X. Angew. Chem., Int. Ed. 2021, 60, 15254.
|
|
(b) Dong Y.-Q.; Wang K.; Zhuo C.-X. ACS Catal. 2022, 12, 11428.
|
|
|
(c) Cao L.-Y.; Wang J.-L.; Wang K.; Wu J.-B.; Wang D.-K.; Peng J.-M.; Bai J.; Zhuo C.-X. J. Am. Chem. Soc. 2023, 145, 2765.
|
|
|
(d) Yu Y.-Z.; Bai J.; Peng J.-M.; Yao J.-S.; Zhuo C.-X. J. Am. Chem. Soc. 2023, 145, 8781.
|
|
|
(e) Dong Y.-Q.; Shi X.-N.; Cao L.-Y.; Bai J.; Zhuo C.-X. Org. Chem. Front. 2023, 10, 3544.
|
|
| [12] |
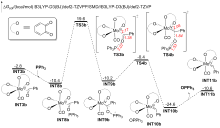
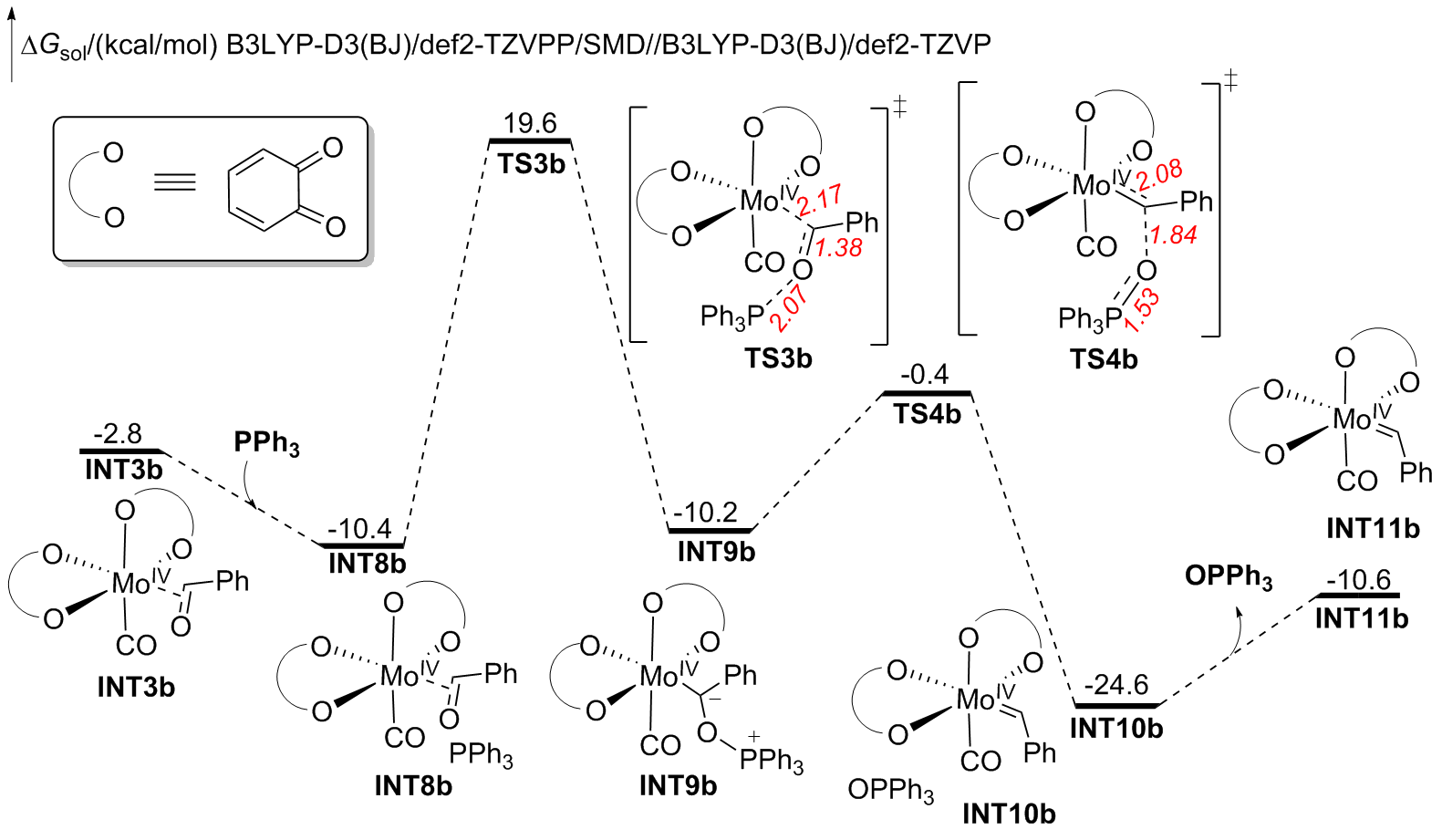
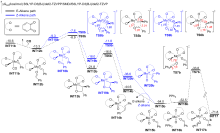
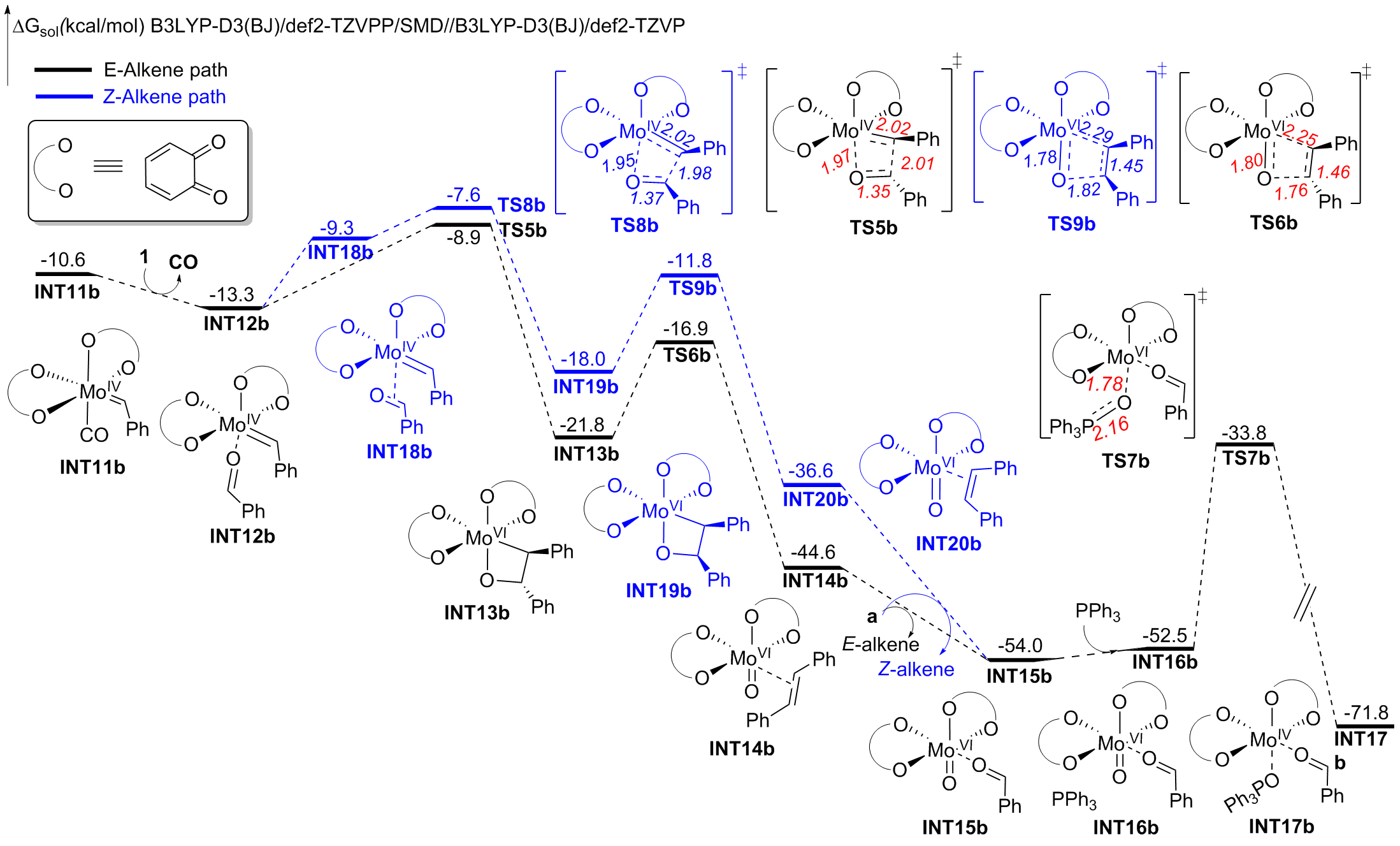
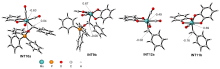
A direct C=O bond cleavage of 1 via path I on equatorial plane to give an oxo-Mo-carbene can also be ruled out due to the even higher activation barrier (Figure S1).
|
| [13] |
A nucleophilic attack via the oxygen atom of the carbonyl group to the carbene moiety of INT12b/INT12b' via TS5b'/TS5b'' could be excluded due to the much higher activation barrier (Figure S3). The Schrock-type carbene character with the negatively charged carbene carbon in INT12b is responsible for the chemo-selectivity.
|
| [14] |
The PPh3 additive assisted deoxygenation of the oxo-Mo-carbene in a reductive elimination style via TS7b' can be excluded due to the much higher activation barrier (Figure S5).
|
| [15] |
(a) Becke A. D. J. Chem. Phys. 1993, 98, 5648.
pmid: 9944570 |
|
(b) Lee C.; Yang W.; Parr R. G. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785.
doi: 10.1103/physrevb.37.785 pmid: 9944570 |
|
|
(c) Grimme S.; Antony J.; Ehrlich S.; Krieg H. J. Chem. Phys. 2010, 132, 154104.
pmid: 9944570 |
|
| [16] |
(a) Schaefer A.; Horn H.; Ahlrichs R. J. Chem. Phys. 1992, 97, 2571.
|
|
(b) Schaefer A.; Huber C.; Ahlrichs R. J. Chem. Phys. 1994, 100, 5829.
|
|
|
(c) Weigend F.; Ahlrichs R. Phys. Chem. Chem. Phys. 2005, 7, 3297.
|
|
| [17] |
(a) Fukui K. J. Phys. Chem. 1970, 74, 4161.
|
|
(b) Fukui K. Acc. Chem. Res. 1981, 14, 363.
|
|
| [18] |
Marenich A. V.; Cramer C. J.; Truhlar D. G. J. Phys. Chem. B 2009, 113, 6378.
|
| [19] |
Martin R. L.; Hay P. J.; Pratt L. R. J. Phys. Chem. A 1998, 102, 3565.
|
| [20] |
(a) Li H.; Jiang J.; Lu G.; Huang F.; Wang Z.-X. Organometallics 2011, 30, 3131.
|
|
(b) Li H.; Wen M.; Wang Z.-X. Inorg. Chem. 2012, 51, 5716.
|
|
|
(c) Wen M.; Huang F.; Lu G.; Wang Z.-X. Inorg. Chem. 2013, 52, 12098.
|
|
|
(d) Qu S.; Dang Y.; Song C.; Wen M.; Huang K.-W.; Wang Z.-X. J. Am. Chem. Soc. 2014, 136, 4974.
|
|
|
(e) Yu J.-L.; Zhang S.-Q.; Hong X. J. Am. Chem. Soc. 2017, 139, 7224.
|
|
| [21] |
Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam N. J.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J. Gaussian 09, revision C.01, Gaussian, Inc., Wallingford, CT, 2010.
|
| [22] |
Legault C. Y. CYLview 1.0b, CYLview 1.0b, Université de Sherbrooke, Sherbrooke, Quebec, Canada, 2009, http://www.Cylview.org
|
| [23] |
Johnson E. R.; Keinan S.; Mori-Sánchez P.; Contreras-Garcia J.; Cohen A. J.; Yang W. J. Am. Chem. Soc. 2010, 132, 6498.
doi: 10.1021/ja100936w pmid: 20394428 |
| [24] |
Lu T.; Chen F. J. Comput. Chem. 2012, 33, 580.
|
| [25] |
Humphrey W.; Dalke A.; Schulten K. J. Mol. Graph. 1996, 14, 33.
doi: 10.1016/0263-7855(96)00018-5 pmid: 8744570 |
| [1] | 蒋镓西, 刘全忠. 乙烯基重氮化合物非金属卡宾机制参与的反应[J]. 有机化学, 2024, 44(9): 2640-2657. |
| [2] | 赵明, 颜瑞, 陈虎. 氮杂环卡宾催化醛类化合物的极性反转[J]. 有机化学, 2024, 44(7): 2204-2215. |
| [3] | 刘晓东, 施世良. ANIPE配体促进的铜催化联烯与亚胺和联硼试剂的不对称碳硼化反应[J]. 有机化学, 2024, 44(6): 1884-1896. |
| [4] | 孙超, 周泉, 李传莹, 王磊. 苯并唑-氮杂环卡宾钯配合物的合成表征及应用[J]. 有机化学, 2024, 44(6): 1957-1966. |
| [5] | 刘岩, 王晓梅, 何林, 李师伍, 赵志飞. 氮杂环卡宾(NHC)催化[3+2]环加成反应高非对映选择性地构建螺氧吲哚二氢呋喃稠合吡唑啉酮化合物[J]. 有机化学, 2024, 44(4): 1301-1310. |
| [6] | 夏登鹏, 罗锦昀, 何林, 蔡志华, 杜广芬. 氮杂环卡宾催化的五氟苯基硫醚的合成[J]. 有机化学, 2024, 44(2): 622-630. |
| [7] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [8] | 李建文, 王涛, 陶晟, 陈飞, 李敏, 刘宁. SBA-15负载的N-杂环卡宾-吡啶钼配合物在二氧化碳转化制备环状碳酸酯中的应用[J]. 有机化学, 2024, 44(10): 3213-3222. |
| [9] | 蔡远林, 吕亚, 聂桂花, 金智超, 池永贵. 氮杂环卡宾催化合成氰基化合物的研究进展[J]. 有机化学, 2023, 43(9): 3135-3145. |
| [10] | 李焕清, 陈兆华, 陈祖佳, 邱琪雯, 张又才, 陈思鸿, 汪朝阳. 基于有机小分子的汞离子荧光探针研究进展[J]. 有机化学, 2023, 43(9): 3067-3077. |
| [11] | 许晓萍, 张翼飞, 莫小渝, 江俊. 铑催化3-重氮吲哚-2-亚胺与吡唑啉酮的C—H官能团化反应制备3-吡唑基吲哚[J]. 有机化学, 2023, 43(7): 2519-2527. |
| [12] | 杨亮茹, 郭梦丽, 袁金伟, 王佳美, 夏宇婷, 肖咏梅, 毛璞. 钳形氮杂环卡宾金属络合物的研究进展[J]. 有机化学, 2023, 43(6): 2002-2025. |
| [13] | 陈志豪, 范奇, 尹标林, 李清江, 王洪根. α-硼取代羰基类化合物的合成进展[J]. 有机化学, 2023, 43(5): 1706-1712. |
| [14] | 张心予, 耿慧慧, 张士磊, 王卫, 陈晓蓓. 一种N-杂环卡宾催化合成氘代苯偶姻的方法[J]. 有机化学, 2023, 43(4): 1510-1516. |
| [15] | 黄华, 李鑫, 苏建科, 宋秋玲. 二氟卡宾参与下从邻乙烯基苯胺出发构建3-取代吲哚酮类化合物[J]. 有机化学, 2023, 43(3): 1146-1156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
