有机化学 ›› 2024, Vol. 44 ›› Issue (10): 3213-3222.DOI: 10.6023/cjoc202405037 上一篇 下一篇
所属专题: 二氧化碳专题合集
研究论文
收稿日期:2024-05-27
修回日期:2024-07-31
发布日期:2024-08-30
基金资助:
Jianwen Li, Tao Wang, Sheng Tao, Fei Chen, Min Li*( ), Ning Liu*(
), Ning Liu*( )
)
Received:2024-05-27
Revised:2024-07-31
Published:2024-08-30
Contact:
*E-mail: Supported by:文章分享

由二氧化碳(CO2)和环氧化物合成环状碳酸酯是CO2利用的有效途径. 尽管各类金属催化剂相继见诸报道, 但依然急需开发一类可回收或再循环的催化剂. 该工作将SBA-15负载的N-杂环卡宾-吡啶钼络合物(Mo@SBA-15)作为一类高效和可循环利用的催化剂应用于CO2和环氧化物合成环状碳酸酯. Mo@SBA-15与四丁基溴化铵(TBAB)组成的双组分催化体系在100 ℃和CO2 (1 MPa)压力下合成环状碳酸酯时, 显示出了较高的催化活性. 此外, Mo@SBA-15重复使用7次未发现明显的活性降低.
李建文, 王涛, 陶晟, 陈飞, 李敏, 刘宁. SBA-15负载的N-杂环卡宾-吡啶钼配合物在二氧化碳转化制备环状碳酸酯中的应用[J]. 有机化学, 2024, 44(10): 3213-3222.
Jianwen Li, Tao Wang, Sheng Tao, Fei Chen, Min Li, Ning Liu. N-Heterocyclic Carbene-Pyridine Molybdenum Complex Supported over SBA-15 for Converting of Carbon Dioxide into Cyclic Carbonates[J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3213-3222.
| Entry | Catalyst (mg) | Cocatalyst (mol%) | T/℃ | p/MPa | Time/h | Yield/% |
|---|---|---|---|---|---|---|
| 1 | None | None | 100 | 1 | 6 | 0 |
| 2 | Mo@SBA-15 (50) | TBAB (3) | 100 | 1 | 6 | 97 |
| 3 | None | TBAB (3) | 100 | 1 | 6 | 62 |
| 4 | Mo@SBA-15 (50) | None | 100 | 1 | 6 | 0 |
| 5 | Mo complex 1a (50) | TBAB (3) | 100 | 1 | 6 | 94 |
| 6 | (3-Chloropropyl) trimethoxysilane (50) | TBAB (3) | 100 | 1 | 6 | 46 |
| 7 | SBA-15 (50) | TBAB (3) | 100 | 1 | 6 | 71 |
| 8 | Mo@SBA-15 (50) | TBAB (3) | 30 | 1 | 6 | 27 |
| 9 | Mo@SBA-15 (50) | TBAB (3) | 60 | 1 | 6 | 52 |
| 10 | Mo@SBA-15 (50) | TBAB (3) | 80 | 1 | 6 | 73 |
| 11 | Mo@SBA-15 (50) | TBAB (3) | 100 | 0.5 | 6 | 70 |
| 12 | Mo@SBA-15 (50) | TBAB (3) | 100 | 1.5 | 6 | 88 |
| 13 | Mo@SBA-15 (20) | TBAB (3) | 100 | 1 | 6 | 87 |
| 14 | Mo@SBA-15 (30) | TBAB (3) | 100 | 1 | 6 | 94 |
| 15 | Mo@SBA-15 (40) | TBAB (3) | 100 | 1 | 6 | 96 |
| 16 | Mo@SBA-15 (30) | TBAB (1) | 100 | 1 | 6 | 49 |
| 17 | Mo@SBA-15 (30) | TBAB (2) | 100 | 1 | 6 | 82 |
| 18 | Mo@SBA-15 (30) | TBAB (3) | 100 | 1 | 2 | 71 |
| 19 | Mo@SBA-15 (30) | TBAB (3) | 100 | 1 | 4 | 82 |
| 20 | Mo@SBA-15 (30) | TBAB (3) | 100 | 1 | 10 | 97 |
| Entry | Catalyst (mg) | Cocatalyst (mol%) | T/℃ | p/MPa | Time/h | Yield/% |
|---|---|---|---|---|---|---|
| 1 | None | None | 100 | 1 | 6 | 0 |
| 2 | Mo@SBA-15 (50) | TBAB (3) | 100 | 1 | 6 | 97 |
| 3 | None | TBAB (3) | 100 | 1 | 6 | 62 |
| 4 | Mo@SBA-15 (50) | None | 100 | 1 | 6 | 0 |
| 5 | Mo complex 1a (50) | TBAB (3) | 100 | 1 | 6 | 94 |
| 6 | (3-Chloropropyl) trimethoxysilane (50) | TBAB (3) | 100 | 1 | 6 | 46 |
| 7 | SBA-15 (50) | TBAB (3) | 100 | 1 | 6 | 71 |
| 8 | Mo@SBA-15 (50) | TBAB (3) | 30 | 1 | 6 | 27 |
| 9 | Mo@SBA-15 (50) | TBAB (3) | 60 | 1 | 6 | 52 |
| 10 | Mo@SBA-15 (50) | TBAB (3) | 80 | 1 | 6 | 73 |
| 11 | Mo@SBA-15 (50) | TBAB (3) | 100 | 0.5 | 6 | 70 |
| 12 | Mo@SBA-15 (50) | TBAB (3) | 100 | 1.5 | 6 | 88 |
| 13 | Mo@SBA-15 (20) | TBAB (3) | 100 | 1 | 6 | 87 |
| 14 | Mo@SBA-15 (30) | TBAB (3) | 100 | 1 | 6 | 94 |
| 15 | Mo@SBA-15 (40) | TBAB (3) | 100 | 1 | 6 | 96 |
| 16 | Mo@SBA-15 (30) | TBAB (1) | 100 | 1 | 6 | 49 |
| 17 | Mo@SBA-15 (30) | TBAB (2) | 100 | 1 | 6 | 82 |
| 18 | Mo@SBA-15 (30) | TBAB (3) | 100 | 1 | 2 | 71 |
| 19 | Mo@SBA-15 (30) | TBAB (3) | 100 | 1 | 4 | 82 |
| 20 | Mo@SBA-15 (30) | TBAB (3) | 100 | 1 | 10 | 97 |
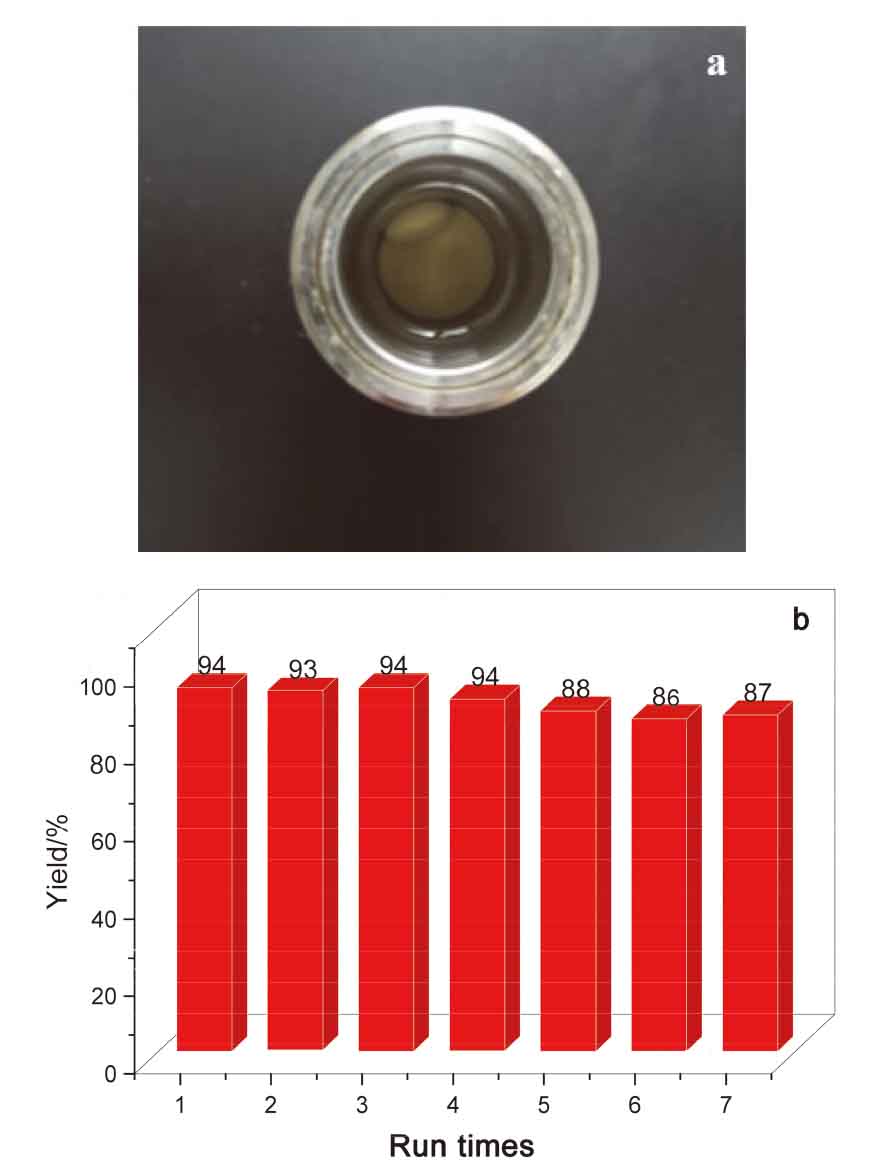
| Entry | Cat. | Nucleophile (mol%) | Epoxide | T/℃ | Time/h | p/MPa | Yield/% | Runs |
|---|---|---|---|---|---|---|---|---|
| 1[ | MoCl5 (0.5 mol%) | PPh3 (3) | PO | r.t. | 168 | 0.1 | 79.0 | 0 |
| 2[ | MoO3 (2 mol%) | [Bu4P]Br (2) | EMO | 100 | 16 | 5 | 98.0 | 0 |
| 3[ | Mo catal. (1 mol%) | TBAB (7.2) | PO | 25 | 24 | 0.1 | 97.5 | 0 |
| 4[ | Mo catal. (0.1 mol%) | Neat | ECH | 100 | 3.5 | 1 | 56.3 | 0 |
| 5[ | Mo catal. (0.5 mol%) | TBAI (2) | EB | 30 | 24 | 0.5 | 94.0 | 0 |
| 6[ | Mo catal. (0.15 mol%) | TBAB (5) | ECH | 70 | 3 | 0.1 | 99.9b | 5 |
| 7[ | Mo catal. (0.1 mmol) | TBAB (1) | SO | 50 | 48 | 0.5 | 99.0b | 3 |
| 8[ | Mo catal. (0.5 mol%) | TBAI (2) | EB | 80 | 20 | 0.5 | 91.0 | 0 |
| 9 (our work) | Mo@SBA-15 (30 mg) | TBAB (3) | EB | 100 | 6 | 1 | 94.0b | 7 |
| Entry | Cat. | Nucleophile (mol%) | Epoxide | T/℃ | Time/h | p/MPa | Yield/% | Runs |
|---|---|---|---|---|---|---|---|---|
| 1[ | MoCl5 (0.5 mol%) | PPh3 (3) | PO | r.t. | 168 | 0.1 | 79.0 | 0 |
| 2[ | MoO3 (2 mol%) | [Bu4P]Br (2) | EMO | 100 | 16 | 5 | 98.0 | 0 |
| 3[ | Mo catal. (1 mol%) | TBAB (7.2) | PO | 25 | 24 | 0.1 | 97.5 | 0 |
| 4[ | Mo catal. (0.1 mol%) | Neat | ECH | 100 | 3.5 | 1 | 56.3 | 0 |
| 5[ | Mo catal. (0.5 mol%) | TBAI (2) | EB | 30 | 24 | 0.5 | 94.0 | 0 |
| 6[ | Mo catal. (0.15 mol%) | TBAB (5) | ECH | 70 | 3 | 0.1 | 99.9b | 5 |
| 7[ | Mo catal. (0.1 mmol) | TBAB (1) | SO | 50 | 48 | 0.5 | 99.0b | 3 |
| 8[ | Mo catal. (0.5 mol%) | TBAI (2) | EB | 80 | 20 | 0.5 | 91.0 | 0 |
| 9 (our work) | Mo@SBA-15 (30 mg) | TBAB (3) | EB | 100 | 6 | 1 | 94.0b | 7 |
| [1] |
Jahandar Lashaki, M.; Khiavi, S.; Sayari, A. Chem. Soc. Rev. 2019, 48, 3320.
doi: 10.1039/c8cs00877a pmid: 31149678 |
| [2] |
de Kleijne, K.; Hanssen, S. V.; van Dinteren, L.; Huijbregts, M. A. J.; van Zelm, R.; de Coninck, H. One Earth 2022, 5, 168.
|
| [3] |
Artz, J.; Muller, T. E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Chem. Rev. 2018, 118, 434.
|
| [4] |
Zhang, J.; Wang, L.; Liu, S.; Li, Z. Angew. Chem., Int. Ed. 2022, 61, e202116982.
|
| [5] |
Meylan, F. D.; Moreau, V.; Erkman, S. J. CO2 Util. 2015, 12, 101.
|
| [6] |
Buttner, H.; Longwitz, L.; Steinbauer, J.; Wulf, C.; Werner, T. Top. Curr. Chem. 2017, 375, 50.
|
| [7] |
Schaffner, B.; Schaffner, F.; Verevkin, S. P.; Borner, A. Chem. Rev. 2010, 110, 4554.
|
| [8] |
Taherimehr, M.; Serta, J. P.; Kleij, A. W.; Whiteoak, C. J.; Pescarmona, P. P. ChemSusChem 2015, 8, 1034.
doi: 10.1002/cssc.201403323 pmid: 25688870 |
| [9] |
Guo, L.; Lamb, K. J.; North, M. Green Chem. 2021, 23, 77.
|
| [10] |
Kotanen, S.; Wirtanen, T.; Mahlberg, R.; Anghelescu-Hakala, A.; Harjunalanen, T.; Willberg-Keyrilainen, P.; Laaksonen, T.; Sarlin, E. J. Appl. Polym. Sci. 2023, 140, e53964.
|
| [11] |
Zhang, X.; Zhao, B.; Fu, S.; Seruya, R. S.; Madey III, J. F.; Bukhryakova, E.; Zhang, F. Macromolecules 2024, 57, 2858.
|
| [12] |
Giri, P.; Barath V, S.; Dhurua, S.; Maity, S.; Gazi, R.; Jana, M. Phys. Chem. Chem. Phys. 2024, 26, 9317.
|
| [13] |
Rehman, A.; Saleem, F.; Javed, F.; Ikhlaq, A.; Ahmad, S. W.; Harvey, A. J. Environ. Chem. Eng. 2021, 9, 105113.
|
| [14] |
Qin, Y.; Guo, H.; Sheng, X.; Wang, X.; Wang, F. Green Chem. 2015, 17, 2853.
|
| [15] |
Jiang, X.; Gou, F.; Fu, X.; Jing, H. J. CO2 Util. 2016, 16, 264.
|
| [16] |
Maeda, C.; Taniguchi, T.; Ogawa, K.; Ema, T. Angew. Chem., Int. Ed. 2015, 54, 134.
|
| [17] |
Chaugule, A. A.; Tamboli, A. H.; Kim, H. Fuel 2017, 200, 316.
|
| [18] |
Wang, B.; Cao, X.; Wang, L.; Meng, X.; Wang, Y.; Sun, W. Inorg. Chem. 2024, 63, 9156.
|
| [19] |
Xiao, L.-F.; Li, F.-W.; Xia, C.-G. Appl. Catal., A 2005, 279, 125.
|
| [20] |
Vyskočilová, E.; Šafařík, D.; Zítová, K.; Vrbková, E.; Dimitrov, R.; Vagenknechtová, A.; Červený, L. Catal. Lett. 2022, 152, 3576.
|
| [21] |
Pappuru, S.; Shpasser, D.; Carmieli, R.; Shekhter, P.; Jentoft, F. C.; Gazit, O. M. ACS Omega 2022, 7, 24656.
|
| [22] |
Mikšovsky, P.; Rauchenwald, K.; Naghdi, S.; Rabl, H.; Eder, D.; Konegger, T.; Bica-Schröder, K. ACS Sustainable Chem. Eng. 2024, 12, 1455.
|
| [23] |
Campisciano, V.; Gruttadauria, M.; Giacalone, F. ChemCatChem 2018, 11, 90.
|
| [24] |
Jayakumar, S.; Li, H.; Chen, J.; Yang, Q. ACS Appl. Mater. Interfaces 2018, 10, 2546.
|
| [25] |
Liu, L.-H.; Liu, L.; Chi, H.-R.; Li, C.-N.; Han, Z.-B. Chem. Commun. 2022, 58, 6417.
|
| [26] |
Helal, A.; Zahir, M. H.; Albadrani, A.; Ekhwan, M. M. Catal. Lett. 2023, 153, 2883.
|
| [27] |
Helal, A.; Alahmari, F.; Usman, M.; Yamani, Z. H. J. Environ. Chem. Eng. 2022, 10, 108061.
|
| [28] |
Qiu, L.-Q.; Li, H.-R.; He, L.-N. Acc. Chem. Res. 2023, 56, 2225.
|
| [29] |
Wang, K.; Li, H.; Yang, L.; Luo, Y.-Z.; Yao, Z.-J. Surf. Interfaces 2024, 45, 103845.
|
| [30] |
Li, J.; Han, Y.; Lin, H.; Wu, N.; Li, Q.; Jiang, J.; Zhu, J. ACS Appl. Mater. Interfaces 2020, 12, 609.
|
| [31] |
Xu, L.; Wang, Y.; Sun, Z.; Chen, Z.; Zhao, G.; Kühn, F. E.; Jia, W.-G.; Yun, R.; Zhong, R. Inorg. Chem. 2024, 63, 1828.
|
| [32] |
Pourhassan, F.; Khalifeh, R.; Eshghi, H. Fuel 2021, 287, 119567.
|
| [33] |
Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G. H.; Chmelka, B. F.; Stucky, G. D. Science 1998, 279, 548.
doi: 10.1126/science.279.5350.548 pmid: 9438845 |
| [34] |
Yang, P.; Zhao, D.; Margolese, D. I.; Chmelka, B. F.; Stucky, G. D. Nature 1998, 396, 152.
|
| [35] |
Dokhaee, Z.; Ghiaci, M.; Farrokhpour, H.; Buntkowsky, G.; Breitzke, H. Ind. Eng. Chem. Res. 2020, 59, 12632.
|
| [36] |
Liu, Y.; Hu, Y. H.; Zhang, J. R.; Zhou, J. S.; Zhang, Z. K.; Wang, L.; Zhang, J.-L. Microporous Mesoporous Mater. 2022, 337, 111873.
|
| [37] |
Pickens, R. N.; Neyhouse, B. J.; Reed, D. T.; Ashton, S. T.; White, J. K. Inorg. Chem. 2018, 57, 11616.
|
| [38] |
Shi, Z.; Su, Q.; Ying, T.; Tan, X.; Deng, L.; Dong, L.; Cheng, W. J. CO2 Util. 2020, 39, 101162.
|
| [39] |
Sankar, M.; Ajithkumar, T. G.; Sankar, G.; Manikandan, P. Catal. Commun. 2015, 59, 201.
|
| [40] |
Yuan, C.; Huang, Z.; Chen, J. Catal. Commun. 2012, 24, 56.
|
| [41] |
Cang, R.; Lu, B.; Li, X.; Niu, R.; Zhao, J.; Cai, Q. Chem. Eng. Sci. 2015, 137, 268.
|
| [42] |
Mohammadi Ziarani, G.; Ebrahimi, Z.; Mohajer, F.; Badiei, A. Res. Chem. Intermed. 2021, 47, 4583.
|
| [43] |
Wang, D.; Guo, X.-Q.; Wang, C.-X.; Wang, Y.-N.; Zhong, R.; Zhu, X.-H.; Cai, L.-H.; Gao, Z.-W.; Hou, X.-F. Adv. Synth. Catal. 2013, 355, 1117.
|
| [44] |
Zhou, L.; Peng, L.; Ji, J.; Ma, W.; Hu, J.; Wu, Y.; Geng, J.; Hu, X. J. CO2 Util. 2022, 58, 101910.
|
| [45] |
Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Catal. Today 1998, 41, 207.
|
| [46] |
Roshan, K. R.; Kathalikkattil, A. C.; Tharun, J.; Kim, D. W.; Won, Y. S.; Park, D. W. Dalton Trans. 2014, 43, 2023.
|
| [47] |
Kathalikkattil, A. C.; Kim, D.-W.; Tharun, J.; Soek, H.-G.; Roshan, R.; Park, D.-W. Green Chem. 2014, 16, 1607.
|
| [48] |
Xie, Y.; Zhang, Z.; Jiang, T.; He, J.; Han, B.; Wu, T.; Ding, K. Angew. Chem., Int. Ed. 2007, 46, 7255.
|
| [49] |
Chen, F.; Tao, S.; Liu, N.; Dai, B. Polyhedron 2021, 196, 114990.
|
| [50] |
Li, C.; Xiong, W.; Zhao, T.; Liu, F.; Cai, H.; Chen, P.; Hu, X. Appl. Catal., B 2023, 324, 122217.
|
| [51] |
Ratzenhofer, M.; Kisch, H. Angew. Chem., Int. Edit. 1980, 19, 317.
|
| [52] |
Tenhumberg, N.; Büttner, H.; Schäffner, B.; Kruse, D.; Blumenstein, M.; Werner, T. Green Chem. 2016, 18, 3775.
|
| [53] |
Chen, J.-H.; Deng, C.-H.; Fang, S.; Ma, J.-G.; Cheng, P. Green Chem. 2018, 20, 989.
|
| [54] |
Kim, Y.; Ryu, S.; Cho, W.; Kim, M.; Park, M. H.; Kim, Y. Inorg. Chem. 2019, 58, 5922.
|
| [55] |
Li, J. W.; Tao, S.; Chen, F.; Li, M.; Liu, N. J. CO2 Util. 2023, 69, 102384.
|
| [56] |
Yu, W.-D.; Zhang, Y.; Han, Y.-Y.; Li, B.; Shao, S.; Zhang, L.-P.; Xie, H.-K.; Yan, J. Inorg. Chem. 2021, 60, 3980.
|
| [57] |
Shi, Z.; Niu, G.; Han, Q.; Shi, X.; Li, M. Mol. Catal. 2018, 461, 10.
|
| [58] |
Cheng, W.; Chen, X.; Sun, J.; Wang, J.; Zhang, S. Catal. Today 2013, 200, 117.
|
| [59] |
Rios Yepes, Y.; Quintero, C.; Osorio Meléndez, D.; Daniliuc, C. G.; Martínez, J.; Rojas, R. S. Organometallics 2019, 38, 469.
|
| [60] |
Desens, W.; Werner, T. Adv. Synth. Catal. 2016, 358, 622.
|
| [61] |
Liu, J.; Yang, G.; Liu, Y.; Zhang, D.; Hu, X.; Zhang, Z. Green Chem. 2020, 22, 4509.
|
| [62] |
Motokucho, S.; Morikawa, H. Chem. Commun. 2020, 56, 10678.
|
| [63] |
Li, Y.-D.; Cui, D.-X.; Zhu, J.-C.; Huang, P.; Tian, Z.; Jia, Y.-Y.; Wang, P.-A. Green Chem. 2019, 21, 5231.
|
| [64] |
Sopeña, S.; Martin, E.; Escudero-Adán, E. C.; Kleij, A. W. ACS Catal. 2017, 7, 3532.
|
| [1] | 夏坤, 张开发, Sher Wali Khan, 阿布力米提•阿布都卡德尔. 二氧化碳参与的三组分偶联反应进展[J]. 有机化学, 2024, 44(5): 1506-1525. |
| [2] | 段东森, 马媛, 刘宇博, 程富, 朱道勇, 王少华. 可见光诱导的二氧化碳对活化烯烃的脱碳羧基化反应[J]. 有机化学, 2024, 44(5): 1675-1685. |
| [3] | 姜晓琳, 王超洋, 武利园, 李跃辉. 含咔唑结构的小分子及聚合物催化二氧化碳转化研究进展[J]. 有机化学, 2024, 44(5): 1423-1444. |
| [4] | 吕帅, 朱钢国, 姚金忠, 周宏伟. 电化学介导的氧化羧化及二氧化碳还原羧化制备羧酸的研究进展[J]. 有机化学, 2024, 44(3): 780-808. |
| [5] | 张澳龙, 杨晗, 程佩栋, 姚阳, 孙松. 可见光促进烯烃与丙二酸酯、CO2的碳-羧化反应研究[J]. 有机化学, 2024, 44(10): 3159-3168. |
| [6] | 袁盼锋, 朱灿明, 孟庆元. 光化学转化二氧化碳合成羧酸化合物的研究进展[J]. 有机化学, 2024, 44(10): 2997-3042. |
| [7] | 徐辉, 蒋慧娴, 阚磊, 徐佩, 朱旭. 可见光诱导甲酸盐参与的炔烃氢羧基化反应[J]. 有机化学, 2024, 44(10): 3241-3248. |
| [8] | 侯静, 黄燕, 李浩, 万远翠, 邵雨, 詹乐武, 王定海, 李斌栋. 二氧化碳自由基阴离子的应用研究进展[J]. 有机化学, 2024, 44(10): 3117-3135. |
| [9] | 李嘉元, 易雅平, 席婵娟. 二氧化碳参与的芳香化合物去芳构化羧化反应研究进展[J]. 有机化学, 2024, 44(10): 3136-3146. |
| [10] | 周宇飞, 贾肖飞. 非均相催化氢甲酰化的串联反应研究进展[J]. 有机化学, 2024, 44(10): 3147-3158. |
| [11] | 石亲, 李臻, 何林, 李玉东, 李跃辉. 硼促进Co催化使用CO2和H2实现仲芳香胺N-甲基化[J]. 有机化学, 2024, 44(10): 3233-3240. |
| [12] | 李文珂, 孙北奇, 张雷, 莫凡洋. 基于自由基机理光催化羧基化反应研究进展[J]. 有机化学, 2024, 44(10): 2961-2996. |
| [13] | 许立锋, 武安国, 于芳羽, 李红茹, 何良年. 可再生能源驱动的CO2基环状碳酸酯合成研究进展[J]. 有机化学, 2024, 44(10): 3091-3105. |
| [14] | 张誉元, 杨昌杰, 唐海涛, 潘英明. 异相催化固定二氧化碳合成羰基衍生物的研究进展[J]. 有机化学, 2024, 44(10): 3077-3090. |
| [15] | 高小童, 钟昱卿, 冯楠, 孙莹, 杨得勇, 周锋. 惰性键与二氧化碳的电化学羧化反应研究[J]. 有机化学, 2024, 44(10): 3043-3062. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||