有机化学 ›› 2025, Vol. 45 ›› Issue (8): 2989-3003.DOI: 10.6023/cjoc202412024 上一篇 下一篇
研究论文
汤敏†, 张斌†, 王秋实, 方超华, 胡立威, 关丽萍*( )
)
收稿日期:2025-01-27
修回日期:2025-02-27
发布日期:2024-05-10
作者简介:†共同第一作者.
基金资助:
Min Tang, Bin Zhang, Qiushi Wang, Chaohua Fang, Liwei Hu, Liping Guan*( )
)
Received:2025-01-27
Revised:2025-02-27
Published:2024-05-10
Contact:
*E-mail:glp730@zjou.edu.cn
About author:These authors contributed equally to this work.
Supported by:文章分享
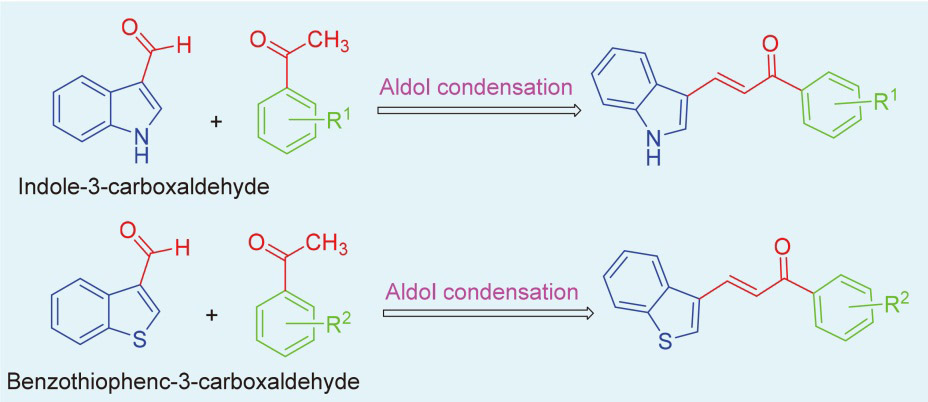
设计合成了31个含有吲哚环和苯并噻吩环的查尔酮衍生物, 并考察了其对胆碱酯酶(ChE)和单胺氧化酶(MAO)的抑制活性. 胆碱酯酶实验的结果显示, 所有化合物对乙酰胆碱酯酶(AChE)的抑制作用较弱, 部分化合物对丁酰胆碱酯酶(BuChE)的抑制效果较为显著, 其中(E)-3-(1H-吲哚-3-基)-1-(间甲苯基)丙-2-烯-1-酮(1a)和(E)-3-(1H-吲哚-3-基)-1-(2-硝基苯基)丙-2-烯-1-酮(1h)对BuChE的抑制效果最好(抑制率分别为85.55%和76.43%). 单胺氧化酶实验结果表明, 部分化合物对单胺氧化酶具有一定的抑制作用. 抑制活性超过50%的[(E)-1-(3,4-二甲基苯基)-3-(1H-吲哚-3-基)丙-2-烯-1-酮(1c), (E)-3-(1H-吲哚-3-基)-1-(3-甲氧基苯基)丙-2-烯-1-酮(1f), (E)-3-(1H-吲哚-3-基)-1-(4-甲氧基苯基)-丙-2-烯-1-酮(1g), (E)-1-(4-羟基苯基)-3-(1H-吲哚-3-基)丙-2-烯-1-酮(1v), (E)-3-(苯并[b]噻吩-3-基)-1-(4-硝基)丙-2-烯-1-酮(1aa)]的MAO-A和MAO-B酶活性被评估, 发现化合物1c, 1f, 1aa对MAO-A和MAO-B都表现出较好的抑制活性. 细胞毒性实验结果显示, 抑制活性较好的化合物对L929细胞无细胞毒性. 此外, 化合物1a、1c和1f的分子对接结果表明, 化合物1a和1f与BuChE以及化合物1c和1f与MAO-A和MAO-B之间存在显著的相互作用.
汤敏, 张斌, 王秋实, 方超华, 胡立威, 关丽萍. 基于包含吲哚环、苯并噻吩环的单胺氧化酶和胆碱酯酶抑制活性的查尔酮衍生物设计、合成及生物活性研究[J]. 有机化学, 2025, 45(8): 2989-3003.
Min Tang, Bin Zhang, Qiushi Wang, Chaohua Fang, Liwei Hu, Liping Guan. Design, Synthesis and Biological Activity Study of Chalcone Derivatives Based on the Inhibitory Activities of Monoamine Oxidase and Cholinesterase Containing Indole Ring and Benzothiophene Ring[J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2989-3003.
| Compound | R | IC50/(µmol•L-1) |
|---|---|---|
| BuChE | ||
| 1a | 3-CH3 | 0.48±0.34 |
| 1f | 3-OCH3 | 24.56±0.12 |
| 1h | 2-NO2 | 47.81±0.92 |
| 1k | 2-F | 41.48±1.45 |
| 1l | 3-F | 36.38±0.23 |
| 1ab | 3-OH | 87.67±3.27 |
| Tacrine | — | 36.38±0.23 |
| Compound | R | IC50/(µmol•L-1) |
|---|---|---|
| BuChE | ||
| 1a | 3-CH3 | 0.48±0.34 |
| 1f | 3-OCH3 | 24.56±0.12 |
| 1h | 2-NO2 | 47.81±0.92 |
| 1k | 2-F | 41.48±1.45 |
| 1l | 3-F | 36.38±0.23 |
| 1ab | 3-OH | 87.67±3.27 |
| Tacrine | — | 36.38±0.23 |
| Compound | R | Inhibition rate/% | IC50/(µmol•L-1) | |||
|---|---|---|---|---|---|---|
| MAO-A | MAO-B | MAO-A | MAO-B | |||
| 1c | 3,4-(CH3)2 | 52.46 | 51.11 | 77.85±1.36 | 24.96±2.33 | |
| 1f | 3-OCH3 | 51.88 | 52.97 | 120.15±5.87 | 81.07±2.41 | |
| 1g | 4-OCH3 | 46.07 | 48.89 | 53.74±0.95 | 104.95±3.46 | |
| 1v | 4-OH | 30.16 | 32.80 | 49.38±1.23 | >150 | |
| 1aa | 4-NO2 | 64.37 | 57.22 | >150 | 51.99±0.76 | |
| Clorgiline | — | 89.35 | — | 47.82±2.74 | — | |
| Pargyline | — | — | 87.65 | — | 5.40±0.21 | |
| Compound | R | Inhibition rate/% | IC50/(µmol•L-1) | |||
|---|---|---|---|---|---|---|
| MAO-A | MAO-B | MAO-A | MAO-B | |||
| 1c | 3,4-(CH3)2 | 52.46 | 51.11 | 77.85±1.36 | 24.96±2.33 | |
| 1f | 3-OCH3 | 51.88 | 52.97 | 120.15±5.87 | 81.07±2.41 | |
| 1g | 4-OCH3 | 46.07 | 48.89 | 53.74±0.95 | 104.95±3.46 | |
| 1v | 4-OH | 30.16 | 32.80 | 49.38±1.23 | >150 | |
| 1aa | 4-NO2 | 64.37 | 57.22 | >150 | 51.99±0.76 | |
| Clorgiline | — | 89.35 | — | 47.82±2.74 | — | |
| Pargyline | — | — | 87.65 | — | 5.40±0.21 | |











| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
doi: 10.1093/ageing/afl027 pmid: 16788077 |
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
doi: 10.1016/bs.pbr.2020.07.016 pmid: 33785136 |
| [12] |
|
| [13] |
doi: 10.1007/s11357-023-01030-x pmid: 38097855 |
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
doi: 10.1097/PRA.0000000000000779 pmid: 38819242 |
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [1] | 田海平, 刘东东, 裴鸿艳, 叶家麟, 郑子锐, 高一星, 李昌兴, 田欢, 张静, 张立新. 新型苯基吡唑类衍生物的设计、合成和杀虫活性研究[J]. 有机化学, 2025, 45(1): 227-239. |
| [2] | 颜逸韬, 陈颖露, 胡涵显, 吴军. 双取代嘧啶-联苯化合物的合成及除草活性和分子作用机制研究[J]. 有机化学, 2025, 45(1): 358-366. |
| [3] | 郑佰峰, 左炀, 陈琼, 吴琼友. 新型苯并噻唑-嘧啶二酮类化合物的设计、合成与除草活性研究[J]. 有机化学, 2024, 44(7): 2371-2376. |
| [4] | 梁国超, 董婷婷, 纪海莹, 王春艳, 宋亚丽, 张伟. 新型3,3'-((4-氯-2H-硫色烯-3-基)亚甲基)双(1H-吲哚)类拓扑异构酶Ⅱ抑制剂的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(6): 1949-1956. |
| [5] | 秦丽清, 林桂汕, 段文贵, 崔玉成, 杨卯芳, 李芳耀, 李典鹏. 新型长叶烯基萘满并N-酰基吡唑化合物的合成、抗增殖活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2024, 44(6): 1967-1977. |
| [6] | 胡懿鸣, 许嘉宇, 汤敏, 刘雅雯, 关丽萍, 金晴昊. 2-(1,3-二氧代异吲哚啉-2-基)-N-苯乙酰胺和2-(3,4-二氢异喹啉-1-基)异吲哚-1,3-二酮类单胺氧化酶(MAO)和胆碱酯酶(ChE)抑制剂的设计、合成和生物活性研究[J]. 有机化学, 2024, 44(6): 1907-1919. |
| [7] | 朱红波, 王吉, 胡炜彦, 周堂, 林芷淇, 张荣平, 耿长安, 陈兴龙. 大狼毒根茎中对胰腺癌SW1990细胞具有毒性的二萜[J]. 有机化学, 2024, 44(6): 1929-1937. |
| [8] | 裴鸿艳, 叶家麟, 王锋, 刘东东, 余裕奎, 张静, 张立新. 新型含哌啶结构的脲嘧啶类化合物的设计合成与除草活性研究[J]. 有机化学, 2024, 44(5): 1592-1605. |
| [9] | 吴思敏, 唐嘉欣, 周于佳, 徐学涛, 张昊星, 王少华. 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024, 44(2): 613-621. |
| [10] | 王锋, 陈钰, 裴鸿艳, 张静, 张立新. 含哌啶的新型1,2,4-噁二唑类衍生物的设计合成及抗真菌活性研究[J]. 有机化学, 2023, 43(8): 2826-2836. |
| [11] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [12] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [13] | 孙昌兴, 张福豪, 张欢, 李鹏辉, 姜林. 新型2-(1-甲基-1H-吡唑-4-基)嘧啶-4-甲酰胺的设计、合成、杀菌活性及分子对接研究[J]. 有机化学, 2023, 43(1): 229-235. |
| [14] | 李蕾, 朱聪聪, 朱全刚, 陈中建, 高希珂. 愈创木薁衍生物的设计合成及其抗氧化、抗炎活性研究[J]. 有机化学, 2022, 42(9): 2906-2913. |
| [15] | 王长凯, 孙腾达, 张学博, 杨新玲, 路星星, 徐欢, 石发胜, 张莉, 凌云. 新型含氟吡唑酰肼类化合物的设计合成与生物活性研究[J]. 有机化学, 2022, 42(5): 1527-1536. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||