有机化学 ›› 2025, Vol. 45 ›› Issue (2): 531-545.DOI: 10.6023/cjoc202407007 上一篇 下一篇
综述与进展
收稿日期:2024-07-03
修回日期:2024-09-02
发布日期:2024-10-10
基金资助:
Yuan She, Shuyu Zhang, Le Wang( )
)
Received:2024-07-03
Revised:2024-09-02
Published:2024-10-10
Contact:
*E-mail: 1561113578@qq.com
Supported by:文章分享

近年来, 内烯烃的烯丙基C—H键胺化反应发展迅速, 该类反应通过C—H键活化的方式高效地将氮源引入内烯烃的烯丙基位点构筑C—N键, 从而合成具有重要生物活性和药物前体的含氮化合物. 由于内烯烃活性较低, 并且在反应过程中存在化学选择性、区域选择性和立体选择性等多重挑战, 因此内烯烃的选择性烯丙基C—H键胺化反应一直是有机化学家的热门研究方向. 该综述主要介绍了近年来部分选择性内烯烃烯丙基C—H键胺化反应, 根据区分不同烯丙基C—H键的方法进行分类, 总结了该领域的研究进展及该反应在合成生物活性分子中的应用潜力.
厍远, 张书宇, 王乐. 内烯烃烯丙基选择性C—H键胺化反应研究进展[J]. 有机化学, 2025, 45(2): 531-545.
Yuan She, Shuyu Zhang, Le Wang. Advances in Selective Allylic C—H Amination of Internal Olefins[J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 531-545.
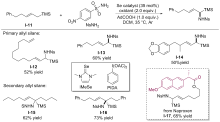





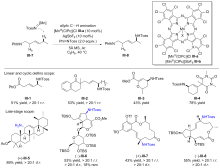



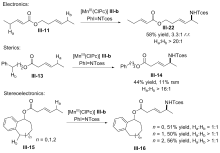



| [1] |
Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257.
doi: 10.1021/jm501100b pmid: 25255204 |
| [2] |
Brown, D. G.; Boström, J. J. Med. Chem. 2016, 59, 4443.
doi: 10.1021/acs.jmedchem.5b01409 pmid: 26571338 |
| [3] |
Park, Y.; Kim, Y.; Chang, S. Chem. Rev. 2017, 117, 9247.
|
| [4] |
Wender, P. A.; Verma, V. A.; Paxton, T. J.; Pillow, T. H. Acc. Chem. Res. 2008, 41, 40.
|
| [5] |
Trost, B. M.; Hansmann, M. M.; Thaisrivongs, D. A. Angew. Chem., Int. Ed. 2012, 51, 4950.
|
| [6] |
Davies, H. M. L.; Mortona, D. Chem. Soc. Rev. 2011, 40, 1857.
doi: 10.1039/c0cs00217h pmid: 21359404 |
| [7] |
Cernak, T.; Dykstra, K. D.; Tyagarajan, S.; Vachalb, P.; Krskab, S. W. Chem. Soc. Rev. 2016, 45, 546.
doi: 10.1039/c5cs00628g pmid: 26507237 |
| [8] |
Pàmies. O.; Margalef. J. S.; Judge. E.; Guiry. P. J.; Moberg. C.; Pericas. M. A. Chem. Rev. 2021, 121, 4373.
doi: 10.1021/acs.chemrev.0c00736 pmid: 33739109 |
| [9] |
Burman, J. S.; Harris, R. J.; Farr, C. M. B.; Bacsa, J.; Blakey, S. B. ACS Catal. 2019, 9, 5474.
doi: 10.1021/acscatal.9b01338 |
| [10] |
Bayeh, L.; Tambar, U. K. ACS Catal. 2017, 7, 8533.
|
| [11] |
Bayeh, L.; Le, P.; Tambar, U. K. Nature 2017, 547, 196.
|
| [12] |
Knecht, T.; Mondal, S.; Ye, J.-H.; Das, M.; Glorius, F. Angew. Chem., Int. Ed. 2019, 58, 7117.
|
| [13] |
Farr, C. M. B.; Kazerouni, A. M.; Park, B.; Poff, C. D.; Won, J.; Sharp, K. R.; Baik, M. H.; Blakey, S. B. J. Am. Chem. Soc. 2020, 142, 13996.
|
| [14] |
Lei, H.; Rovis, T. Nat. Chem. 2020, 12, 725.
|
| [15] |
Maloney, T. P.; Berman, J. L.; Michael, F. E. Angew. Chem., Int. Ed. 2022, 61, e202210109.
|
| [16] |
Lin, S.; Liu, Y.; Gao, K.-Y.; Chen, Z.-H.; Qian, J.; Liu, X.-B.; Li, Q.; Wang, H. ACS Catal. 2024, 14, 8865.
|
| [17] |
Liu, Y.; Chen, Z.-H.; Li, Y.; Qian, J.; Li, Q.-J.; Wang, H.-G. J. Am. Chem. Soc. 2022, 144, 14380.
|
| [18] |
Wang, L.; Wang, C.-L.; Li, Z.-H.; Lian, P.-F.; Kang, J.-C.; Zhou J.; Hao, Y.; Liu, R.-X.; Bai, H.-Y.; Zhang, S.-Y. Nat. Commun. 2024, 15, 1483.
doi: 10.1038/s41467-024-45875-y pmid: 38374064 |
| [19] |
Ide, T.; Feng, K.; Dixon, C. F.; Teng, D.; Clark, J. R.; Han, W.; Wendell, C. I.; Koch, V.; White, M. C. J. Am. Chem. Soc. 2021, 143, 14969.
|
| [20] |
Yang, B.; Liu, X.; Yu, A.; Yang, Q.; Wang, Y. ACS Catal. 2022, 12, 13411.
|
| [21] |
Cheung, K. P. S.; Fang, J.; Mukherjee, K.; Mihranyan, A.; Gevorgyan, V. Science 2022, 378, 1207.
|
| [1] | 张经明, 何智涛. 过渡金属催化远程二烯的不对称迁移烯丙位碳氢键官能团化[J]. 有机化学, 2025, 45(2): 592-601. |
| [2] | 林恩泽, 李必杰. 基于碳氢键断裂的金属催化的内烯烃不对称氢芳基化进展[J]. 有机化学, 2025, 45(2): 546-558. |
| [3] | 王家晟, 王泽树, 何卫民, 叶龙武. 邻炔基苯胺氢胺化合成轴手性吲哚研究进展[J]. 有机化学, 2024, 44(6): 1786-1792. |
| [4] | 李梦帆, 程旭. 烯丙基芳香化合物的电化学选择性氧化酯化[J]. 有机化学, 2024, 44(3): 1005-1012. |
| [5] | 张鑫伟, 林水侦, 黄晓雷. 钯催化SO2插入的烯丙基酯与芳基碘化物的还原偶联反应[J]. 有机化学, 2024, 44(11): 3456-3466. |
| [6] | 何姮, 吕兰兰, 刘建全, 王香善. 铜催化苯并呋喃并嘧啶并异吲哚衍生物的合成[J]. 有机化学, 2024, 44(11): 3427-3436. |
| [7] | 秦浩, 侯传金, 梁丁化, 何心伟, 李玲, 胡向平. 手性P,N,N-配体/钯催化的不对称烯丙基取代反应[J]. 有机化学, 2024, 44(1): 282-290. |
| [8] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [9] | 宋晓, 卿晶, 黎君, 贾雪雷, 吴福松, 黄均荣, 金剑, 游恒志. 铜催化格氏试剂的不对称烯丙基烷基化连续流反应[J]. 有机化学, 2023, 43(9): 3174-3179. |
| [10] | 丁俊, 史啸坤, 郝宇, 白贺元, 张书宇. 银催化的β,γ-不饱和酰胺的不对称γ-胺化反应[J]. 有机化学, 2023, 43(8): 2946-2952. |
| [11] | 孙丽, 宋国欣, 韩家乐, 李继玉, 赵月, 杨璐华, 张峰, 赵坤, 毛比明. Morita-Baylis-Hillman加合物和N-羟基邻苯二甲酰亚胺的电化学烯丙基烷基化形成C(sp3)—C(sp3)键[J]. 有机化学, 2023, 43(4): 1574-1583. |
| [12] | 刘春阳, 李燕, 张前. 铜催化环状烯烃烯丙位C(sp3)—H磺酰化反应研究[J]. 有机化学, 2023, 43(3): 1091-1101. |
| [13] | 郭萍, 周勇, 赵杰. 多取代烯烃的Z∶E高选择性合成制备[J]. 有机化学, 2023, 43(3): 855-872. |
| [14] | 陈宇亮, 贺凤开, 王思云, 贾鼎成, 刘亚群, 黄毅勇. 手性磷酸催化α-全碳季碳醛的不对称烯丙基化动力学拆分[J]. 有机化学, 2023, 43(12): 4294-4302. |
| [15] | 唐朵朵, 黄丹凤, 王克虎, 马虎, 冯杨, 任园园, 王君姣, 胡雨来. 锡粉促进下1,3-二取代-1,3-二氢异苯并呋喃化合物的合成[J]. 有机化学, 2023, 43(12): 4227-4238. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||