有机化学 ›› 2024, Vol. 44 ›› Issue (3): 1005-1012.DOI: 10.6023/cjoc202309019 上一篇 下一篇
研究论文
收稿日期:2023-09-19
修回日期:2023-11-29
发布日期:2024-04-02
基金资助:Received:2023-09-19
Revised:2023-11-29
Published:2024-04-02
Contact:
*E-mail: chengxu@nju.edu.cn
Supported by:文章分享

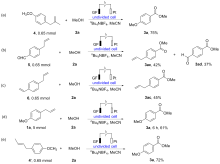
报道了烯丙基芳基化合物的电化学氧化酯化方法, 通过同时活化苄位+烯丙位的C—H和C—C键, 合成了一系列苯甲酸酯类化合物. 本反应展现了良好的化学选择性, 优先在苄位+烯丙位发生氧化, 而其它的苄位C—H键则保持稳定. 该反应在中性条件下进行, 无需酸碱添加剂, 可以适用叔醇酯的构建, 同时避免酯和醇存在下的转移酯化副反应.
李梦帆, 程旭. 烯丙基芳香化合物的电化学选择性氧化酯化[J]. 有机化学, 2024, 44(3): 1005-1012.
Mengfan Li, Xu Cheng. Chemoselective Electro-oxidation of Allyl Arene to Ester[J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 1005-1012.
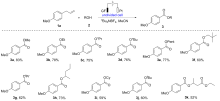
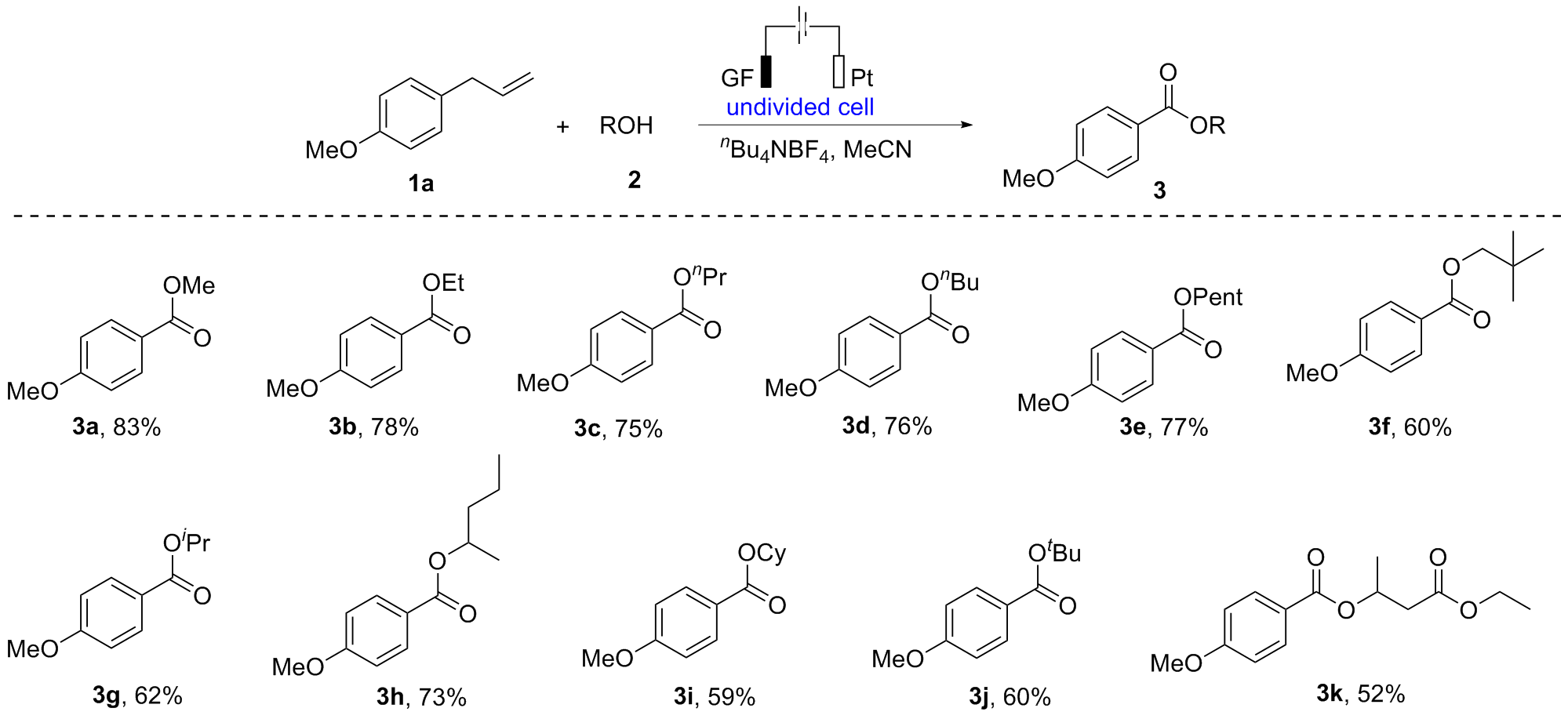
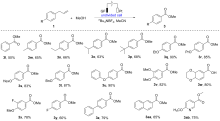
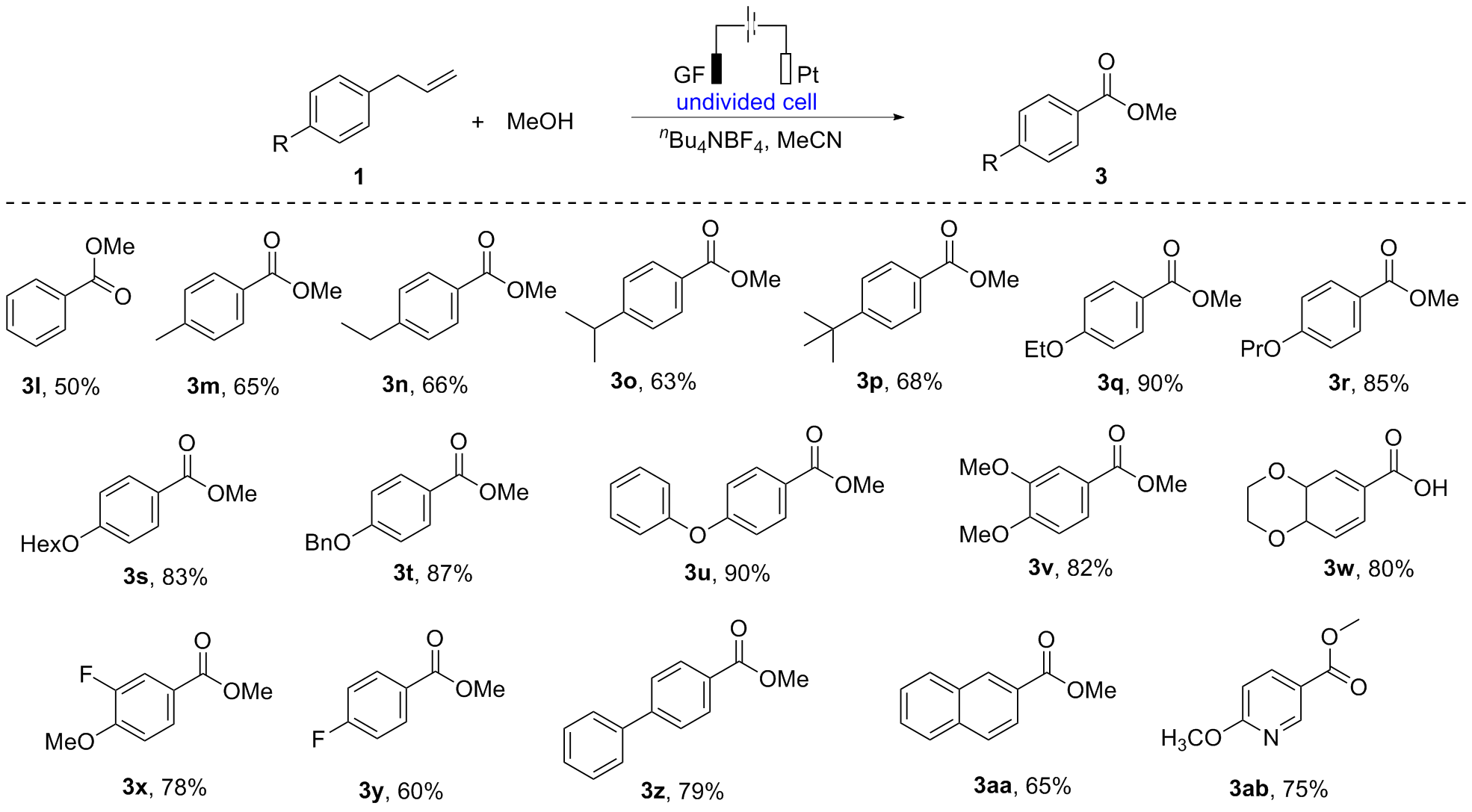
| Entry | Electrolyte | Electrodes | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | nBu4NBF4 | GF anode, GF cathode | MeCN | 33 |
| 2 | nBu4NBF4 | GF anode, Pt cathode | MeCN | 89 (83) |
| 3 | nBu4NClO4 | GF anode, Pt cathode | MeCN | 13 |
| 4 | nBu4NOH | GF anode, Pt cathode | MeCN | 8 |
| 5 | nBu4NBr | GF anode, Pt cathode | DMF | 40 |
| 6 | nBu4NBF4 | GF anode, Pt cathode | Acetone | 47 |
| 7c | nBu4NBF4 | GF anode, Pt cathode | MeCN | 21 |
| 8d | nBu4NBF4 | GF anode, Pt cathode | MeCN | 83 |
| 9e | nBu4NBF4 | GF anode, Pt cathode | MeCN | 63 |
| 10f | nBu4NBF4 | GF anode, Pt cathode | MeCN | 35 |
| 11g | nBu4NBF4 | GF anode, Pt cathode | MeCN | 60 |
| 12h | nBu4NBF4 | GF anode, Pt cathode | MeCN | Trace |
| 13 | nBu4NBF4 | Pt anode, Pt cathode | MeCN | 17 |
| 14 | nBu4NBF4 | Graphite plate anode, Pt cathode | MeCN | Trace |
| Entry | Electrolyte | Electrodes | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | nBu4NBF4 | GF anode, GF cathode | MeCN | 33 |
| 2 | nBu4NBF4 | GF anode, Pt cathode | MeCN | 89 (83) |
| 3 | nBu4NClO4 | GF anode, Pt cathode | MeCN | 13 |
| 4 | nBu4NOH | GF anode, Pt cathode | MeCN | 8 |
| 5 | nBu4NBr | GF anode, Pt cathode | DMF | 40 |
| 6 | nBu4NBF4 | GF anode, Pt cathode | Acetone | 47 |
| 7c | nBu4NBF4 | GF anode, Pt cathode | MeCN | 21 |
| 8d | nBu4NBF4 | GF anode, Pt cathode | MeCN | 83 |
| 9e | nBu4NBF4 | GF anode, Pt cathode | MeCN | 63 |
| 10f | nBu4NBF4 | GF anode, Pt cathode | MeCN | 35 |
| 11g | nBu4NBF4 | GF anode, Pt cathode | MeCN | 60 |
| 12h | nBu4NBF4 | GF anode, Pt cathode | MeCN | Trace |
| 13 | nBu4NBF4 | Pt anode, Pt cathode | MeCN | 17 |
| 14 | nBu4NBF4 | Graphite plate anode, Pt cathode | MeCN | Trace |

| [1] |
(a) Liang Y.-F.; Jiao N. Acc. Chem. Res. 2017, 50, 1640.
doi: 10.1021/acs.accounts.7b00108 |
|
(b) White M. C.; Zhao J. J. Am. Chem. Soc. 2018, 140, 13988.
doi: 10.1021/jacs.8b05195 |
|
|
(c) Dalton T.; Faber T.; Glorius F. ACS Cent. Sci. 2021, 7, 245.
doi: 10.1021/acscentsci.0c01413 |
|
| [2] |
(a) Shi S.-H.; Liang Y.; Jiao N. Chem. Rev. 2021, 121, 485.
doi: 10.1021/acs.chemrev.0c00335 pmid: 33017147 |
|
(b) Cheng X.; Lei A.; Mei T.-S.; Xu H.-C.; Xu K.; Zeng C. CCS Chem. 2022, 4, 1120.
doi: 10.31635/ccschem.021.202101451 pmid: 33017147 |
|
|
(c) Li M.; Cheng X. Isr. J. Chem. 2023, n/a, e202300067.
pmid: 33017147 |
|
|
(d) Zhang P.; Wang T.; Gong J. CCS Chem. 2023, 5, 1028.
doi: 10.31635/ccschem.023.202202643 pmid: 33017147 |
|
| [3] |
(a) Tang S.; Liu Y.; Lei A. Chem 2018, 4, 27.
doi: 10.1016/j.chempr.2017.10.001 |
|
(b) Meyer T. H.; Finger L. H.; Gandeepan P.; Ackermann L. Trends Chem. 2019, 1, 63.
doi: 10.1016/j.trechm.2019.01.011 |
|
|
(c) Yuan Y.; Lei A. Acc. Chem. Res. 2019, 52, 3309.
doi: 10.1021/acs.accounts.9b00512 |
|
|
(d) Qiu Y.; Zhu C.; Stangier M.; Struwe J.; Ackermann L. CCS Chem. 2021, 3, 1529.
doi: 10.31635/ccschem.020.202000365 |
|
| [4] |
(a) Marko J. A.; Durgham A.; Bretz S. L.; Liu W. Chem. Commun. 2019, 55, 937.
doi: 10.1039/C8CC08768G |
|
(b) Sun Y.; Li X.; Yang M.; Xu W.; Xie J.; Ding M. Green Chem. 2020, 22, 7543.
doi: 10.1039/D0GC01871F |
|
|
(c) Atkins A. P.; Rowett A. C.; Heard D. M.; Tate J. A.; Lennox A. J. J. Org. Lett. 2022, 24, 5105.
doi: 10.1021/acs.orglett.2c01930 |
|
|
(d) Hoque M. A.; Twilton J.; Zhu J.; Graaf M. D.; Harper K. C.; Tuca E.; DiLabio G. A.; Stahl S. S. J. Am. Chem. Soc. 2022, 144, 15295.
doi: 10.1021/jacs.2c05974 |
|
|
(e) Chen T.-S.; Long H.; Gao Y.; Xu H.-C. Angew. Chem., Int. Ed. 2023, e202310138.
|
|
| [5] |
Seidler J.; Strugatchi J.; Gärtner T.; Waldvogel S. R. MRS Energy Sustainability 2021, 7, 42.
doi: 10.1557/mre.2020.42 |
| [6] |
(a) Horn E. J.; Rosen B. R.; Chen Y.; Tang J.; Chen K.; Eastgate M. D.; Baran P. S. Nature 2016, 533, 77.
doi: 10.1038/nature17431 |
|
(b) Kazerouni A. M.; McKoy Q. A.; Blakey S. B. Chem. Commun. 2020, 56, 13287.
doi: 10.1039/D0CC05554A |
|
| [7] |
Yu X.; Zhao Z.; Zhu L.; Tan S.; Fu W.; Wang L.; An Y. Mol. Catal. 2022, 519, 112152.
|
| [8] |
(a) Martinez-Erro S.; Sanz-Marco A.; Bermejo Gómez A.; Vázquez-Romero A.; Ahlquist M. S. G.; Martín-Matute B. J. Am. Chem. Soc. 2016, 138, 13408.
doi: 10.1021/jacs.6b08350 pmid: 27636591 |
|
(b) Hayashi R.; Ando K.; Udagawa T.; Sai M. Adv. Synth. Catal. 2023, 365, 826.
doi: 10.1002/adsc.v365.6 pmid: 27636591 |
|
|
(c) Yayla H. G.; Wang H.; Tarantino K. T.; Orbe H. S.; Knowles R. R. J. Am. Chem. Soc. 2016, 138, 10794.
doi: 10.1021/jacs.6b06517 pmid: 27636591 |
|
| [9] |
(a) Kang J.-C.; Tu Y.-Q.; Dong J.-W.; Chen C.; Zhou J.; Ding T.-M.; Zai J.-T.; Chen Z.-M.; Zhang S.-Y. Org. Lett. 2019, 21, 2536.
doi: 10.1021/acs.orglett.9b00263 |
|
(b) Chen C.; Kang J.-C.; Mao C.; Dong J.-W.; Xie Y.-Y.; Ding T.-M.; Tu Y.-Q.; Chen Z.-M.; Zhang S.-Y. Green Chem. 2019, 21, 4014.
doi: 10.1039/C9GC01152H |
| [1] | 叶丹锋, 徐冰, 万云辉. 叔丁醇锂催化N-苄基-N-叔丁氧羰基酰胺与糖的酯化反应[J]. 有机化学, 2024, 44(9): 2924-2932. |
| [2] | 于兆锦, 张展, 王海洋, 王波, 付光明, 张瑛, 杨晓朋, 董爱君, 姬小明. 乙基麦芽酚丁二酸双酯的合成、热解及抗氧化研究[J]. 有机化学, 2024, 44(5): 1526-1534. |
| [3] | 万云辉, 杨福美, 陈明瀚, 孙德立, 叶丹锋. 无过渡金属催化的N-苄基-N-叔丁氧羰基酰胺与不饱和醇的酯化反应[J]. 有机化学, 2024, 44(4): 1293-1300. |
| [4] | 方新月, 黄雅雯, 胡新伟, 阮志雄. 电化学修饰氨基酸和多肽类化合物的研究进展[J]. 有机化学, 2024, 44(3): 903-926. |
| [5] | 何蔺恒, 夏稳, 周玉祥, 于贤勇. 电催化N-芳基甘氨酸和苯并[e][1,2,3]噁噻嗪-2,2-二氧化物的串联脱羧环化反应[J]. 有机化学, 2024, 44(3): 997-1004. |
| [6] | 王竣永, 李娜, 柯杰, 何川. 电化学硅基化反应的研究进展[J]. 有机化学, 2024, 44(3): 927-939. |
| [7] | 孙雪, 颜廷涛, 闫克鲁, 杨建静, 文江伟. 电化学促使α-重氮酯的磷酸化构筑亚膦酸腙[J]. 有机化学, 2024, 44(3): 1013-1020. |
| [8] | 李章健, 王振华, 郭剑峰, 方萍, 马聪, 刘润华, 梅天胜. 电化学促进2,2,6,6-四甲基哌啶氧化物(TEMPO)介导的甘氨酸衍生物氧化脱氢Povarov/串联反应[J]. 有机化学, 2024, 44(3): 940-950. |
| [9] | Hasil Aman, 常瑞, 叶俊涛. 氧化型光电催化促进的C—H键官能团化反应研究进展[J]. 有机化学, 2024, 44(3): 728-747. |
| [10] | 吕帅, 朱钢国, 姚金忠, 周宏伟. 电化学介导的氧化羧化及二氧化碳还原羧化制备羧酸的研究进展[J]. 有机化学, 2024, 44(3): 780-808. |
| [11] | 杨帆, 方婷, 杨桂春, 高梦. 亚硝基苯参与的电化学串联环化反应构建喹啉/吡咯[J]. 有机化学, 2024, 44(3): 1021-1030. |
| [12] | 黄健, 张文珍. 碳氮键参与的电化学阴极还原反应研究进展[J]. 有机化学, 2024, 44(3): 825-839. |
| [13] | 陈远航, 何劲宇, 张博, 王延钊, 孔令轩, 钱伟烽, 王娜娜, 段闻喜, 欧阳妍妍, 朱翠菊, 徐浩. 不对称电化学有机合成[J]. 有机化学, 2024, 44(3): 748-779. |
| [14] | 周兰, 何红, 杨德巧, 侯中伟, 王磊. N-苄基丙烯酰胺的电化学三氟甲基化/螺环化合成三氟甲基取代2-氮杂螺[4.5]癸烷[J]. 有机化学, 2024, 44(3): 981-988. |
| [15] | 吴际伟, 何俊, 王晶晶, 李丽霞, 徐采玉, 周洁, 李子荣, 许华建. 电化学氧化α-酮酸与邻氨基苄胺的脱羧环化反应[J]. 有机化学, 2024, 44(3): 972-980. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
