有机化学 ›› 2025, Vol. 45 ›› Issue (12): 4405-4416.DOI: 10.6023/cjoc202504009 上一篇 下一篇
研究论文
温吉林a, 郭鹏a, 蒲国良a, 满雪玉b,*( ), 贺春阳a,*(
), 贺春阳a,*( )
)
收稿日期:2025-04-08
修回日期:2025-06-05
发布日期:2025-07-11
通讯作者:
满雪玉, 贺春阳
基金资助:
Jilin Wena, Peng Guoa, Guoliang Pua, Xueyu Manb,*( ), Chun-Yang Hea,*(
), Chun-Yang Hea,*( )
)
Received:2025-04-08
Revised:2025-06-05
Published:2025-07-11
Contact:
Xueyu Man, Chun-Yang He
Supported by:文章分享

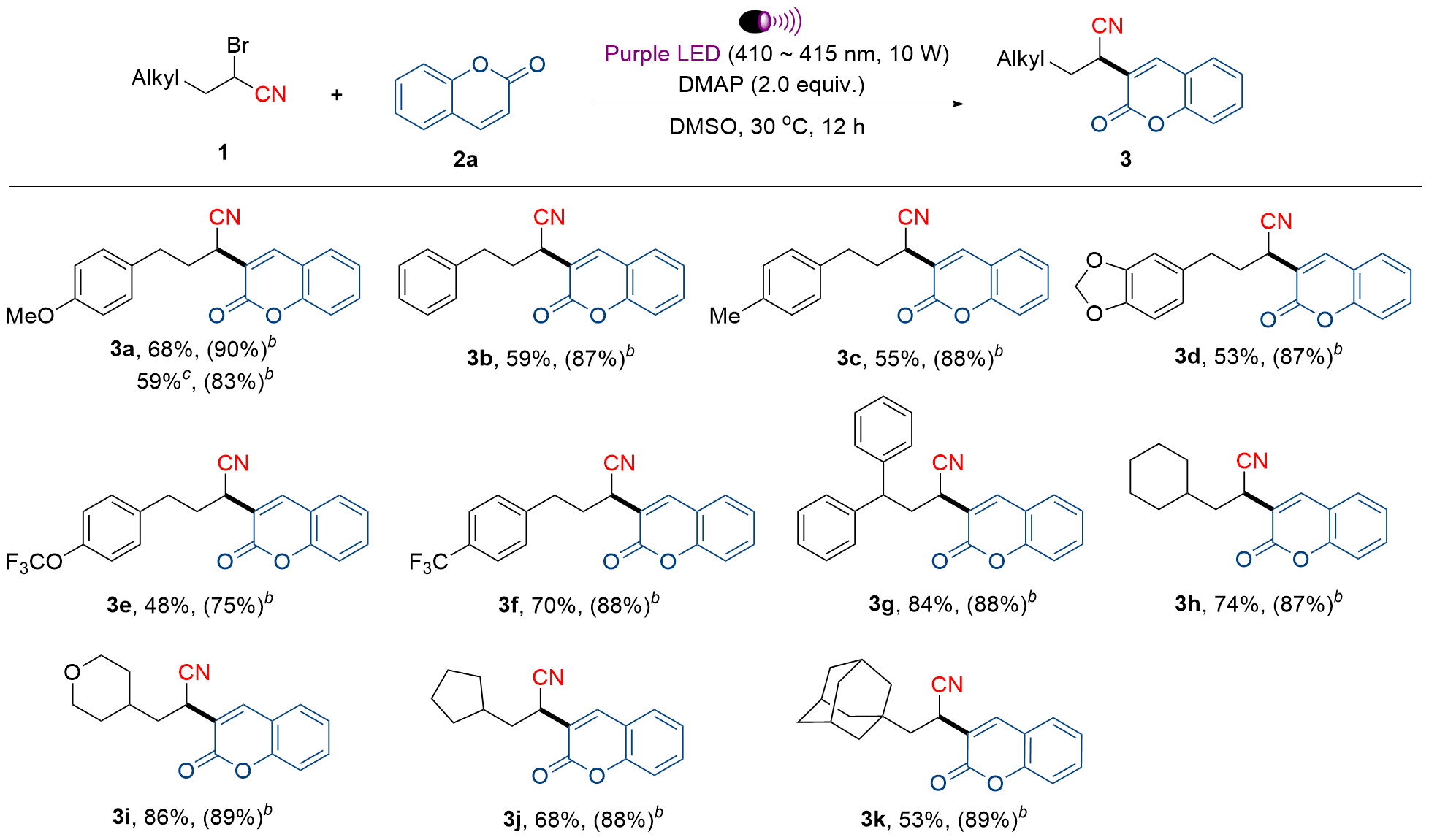
开发了一种由卤键促进的 2-溴丙腈衍生物与香豆素/喹啉酮类化合物的交叉偶联反应. 该方法反应体系简单, 反应条件温和, 并能以中等至良好的收率获得目标产物. 目标化合物中的氰基官能团可在温和条件下高效转化为 2H-四唑衍生物. 使用酯类类似物进行了对比研究, 结果显示其反应活性相较于氰基对应物显著降低, 这凸显了氰基在该转化过程中的重要作用. 初步细胞实验表明, 化合物4b选择性地杀伤MDA-MB-231细胞, 其IC50值为(22.8±1.3) μmol/L, 其他多数化合物对肿瘤细胞和HL-7702正常细胞均表现出无毒或者较低毒性. 上述初步的活性筛选结果为后续抗氧化、抗菌等其他生物活性的深入研究奠定了基础.
温吉林, 郭鹏, 蒲国良, 满雪玉, 贺春阳. 卤键促进的2-溴丙腈衍生物与香豆素/喹啉酮的交叉偶联反应: 合成与转化[J]. 有机化学, 2025, 45(12): 4405-4416.
Jilin Wen, Peng Guo, Guoliang Pu, Xueyu Man, Chun-Yang He. Halogen-Bond-Promoted Direct Cross-Coupling of 2-Bromo- propionitrile Derivatives with Coumarins/Quinolinones: Synthesis and Transformation[J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4405-4416.
| Entry | Electron donor | LED light/nm | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | DMAP | 410~415 | DMSO | 52 |
| 2 | DMAP | 410~415 | DCM | N. D. |
| 3 | DMAP | 410~415 | THF | N. D. |
| 4 | DMAP | 410~415 | CH3OH | N. D. |
| 5 | DMAP | 410~415 | DMF | 31 |
| 6 | DMAP | 410~415 | CH3CN | N. D. |
| 7 | PPh3 | 410~415 | DMSO | N. D. |
| 8 | HE | 410~415 | DMSO | N. D. |
| 9 | PhOH | 410~415 | DMSO | N. D. |
| 10 | Et3N | 410~415 | DMSO | 44 |
| 11 | DBU | 410~415 | DMSO | Trace |
| 12 | DIPEA | 410~415 | DMSO | 16 |
| 13 | DMAP | 390~395 | DMSO | 38 |
| 14c | DMAP | 440~445 | DMSO | 49 |
| 15d | DMAP | 410~415 | DMSO | 41 |
| 16e | DMAP | 410~415 | DMSO | 58 |
| 17f | DMAP | 410~415 | DMSO | 68 |
| 18g | DMAP | 410~415 | DMSO | 69 |
| 19 | — | 410~415 | DMSO | N.D. |
| 20h | DMAP | — | DMSO | N.D. |
| Entry | Electron donor | LED light/nm | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | DMAP | 410~415 | DMSO | 52 |
| 2 | DMAP | 410~415 | DCM | N. D. |
| 3 | DMAP | 410~415 | THF | N. D. |
| 4 | DMAP | 410~415 | CH3OH | N. D. |
| 5 | DMAP | 410~415 | DMF | 31 |
| 6 | DMAP | 410~415 | CH3CN | N. D. |
| 7 | PPh3 | 410~415 | DMSO | N. D. |
| 8 | HE | 410~415 | DMSO | N. D. |
| 9 | PhOH | 410~415 | DMSO | N. D. |
| 10 | Et3N | 410~415 | DMSO | 44 |
| 11 | DBU | 410~415 | DMSO | Trace |
| 12 | DIPEA | 410~415 | DMSO | 16 |
| 13 | DMAP | 390~395 | DMSO | 38 |
| 14c | DMAP | 440~445 | DMSO | 49 |
| 15d | DMAP | 410~415 | DMSO | 41 |
| 16e | DMAP | 410~415 | DMSO | 58 |
| 17f | DMAP | 410~415 | DMSO | 68 |
| 18g | DMAP | 410~415 | DMSO | 69 |
| 19 | — | 410~415 | DMSO | N.D. |
| 20h | DMAP | — | DMSO | N.D. |


| [1] |
(a)
doi: 10.1021/jacs.1c08382 |
|
(b)
doi: 10.1016/j.biopha.2024.117206 |
|
|
(c)
doi: 10.1016/j.bmc.2016.03.004 |
|
|
(d)
doi: 10.1016/j.ejphar.2024.176635 |
|
|
(e)
doi: 10.1016/j.bmc.2013.12.061 |
|
| [2] |
(a)
doi: 10.1039/C5CC03160E pmid: 36876451 |
|
(b)
doi: 10.1039/C8GC03285H pmid: 36876451 |
|
|
(c)
doi: 10.1002/chem.v23.58 pmid: 36876451 |
|
|
(d)
doi: 10.1039/d3ob00062a pmid: 36876451 |
|
|
(e)
pmid: 36876451 |
|
| [3] |
(a)
doi: 10.1021/ol502237a pmid: 32050047 |
|
(b)
doi: 10.1021/acs.orglett.0c00891 pmid: 32050047 |
|
|
(c)
doi: 10.1039/C4QO00007B pmid: 32050047 |
|
|
(d)
doi: 10.1021/acs.joc.9b03070 pmid: 32050047 |
|
|
(e)
doi: 10.1002/anie.201915953 pmid: 32050047 |
|
| [4] |
(a)
pmid: 16302799 |
|
(b)
doi: 10.1016/j.bmc.2010.04.009 pmid: 16302799 |
|
| [5] |
(a)
doi: 10.1016/j.ejmech.2022.114891 |
|
(b)
doi: 10.1021/acs.jmedchem.3c01268 |
|
| [6] |
doi: 10.1021/acs.jmedchem.1c00339 |
| [7] |
(a)
doi: 10.1016/j.ejmech.2019.111587 |
|
(b)
doi: 10.1021/jm070909t |
|
| [8] |
(a)
doi: 10.1016/j.ejmech.2019.111900 pmid: 9003523 |
|
(b)
pmid: 9003523 |
|
| [9] |
(a)
|
|
(b)
doi: 10.1016/j.bmc.2010.04.009 |
|
| [10] |
For recent methods of alkylating coumarin, see: (a)
doi: 10.1021/acs.joc.2c02679 |
|
(b)
doi: 10.1039/c8nj06410e |
|
|
(c)
doi: 10.1016/j.tetlet.2022.153720 |
|
|
(d)
doi: 10.1021/acs.orglett.9b00327 |
|
|
(e)
doi: 10.1002/adsc.v364.1 |
|
|
(f)
doi: 10.1039/D1GC03992J |
|
|
(g)
doi: 10.1021/acs.orglett.4c00717 |
|
|
(h)
doi: 10.1039/D4QO00484A |
|
|
(i)
doi: 10.1002/adsc.v361.17 |
|
|
(j)
doi: 10.1039/C9CC09517A |
|
|
(k)
doi: 10.1039/D4QO00966E |
|
| [11] |
(a)
doi: 10.1021/acs.jmedchem.6b01431 |
|
(b)
|
|
|
(c)
doi: 10.1002/adfm.v33.26 |
|
|
(d)
doi: 10.1021/am3010412 |
|
| [12] |
(a)
doi: 10.1016/j.bmcl.2006.06.007 pmid: 30059123 |
|
(b)
doi: 10.1021/acs.joc.8b01508 pmid: 30059123 |
|
|
(c)
doi: 10.1016/j.cclet.2022.03.096 pmid: 30059123 |
|
|
(d)
doi: 10.1021/jacs.4c03618 pmid: 30059123 |
|
|
(e)
doi: 10.1039/c8ob01513a pmid: 30059123 |
|
| [13] |
doi: 10.1021/acs.chemrev.5b00484 |
| [1] | 谭紫云, 杨新, 龚绍峰, 杨慧翎, 谢永燕, 张静雅, 冯浙泰, 李文艺, 肖新生. 非均相铜催化的叔胺与炔烃需氧氧化交叉偶联反应[J]. 有机化学, 2026, 46(1): 156-166. |
| [2] | 梁家瑞, 李金兰, 蒋云, 姚秋丽, 徐应淑, 王安俊. 可见光驱动下卤键诱导的α-碘甲基膦酸二乙酯与香豆素/喹啉酮的直接偶联反应[J]. 有机化学, 2026, 46(1): 135-145. |
| [3] | 祝辉, 吴鹏, 钟晨鸣, 李舒铭, 林钢, 刘雪粉, 罗书平. 供体-受体-供体(D-A-D)型芳香酮设计合成与光催化C(sp3)—H偶联反应性能研究[J]. 有机化学, 2025, 45(9): 3441-3449. |
| [4] | 贾晨旭, 安聪好, 黄军. 铜催化C—O偶联制备间苯氧基苯甲醛[J]. 有机化学, 2025, 45(9): 3412-3419. |
| [5] | 许文, 罗美明, 曾小明. 铬催化三氟甲基烯烃的还原交叉偶联[J]. 有机化学, 2025, 45(9): 3401-3411. |
| [6] | 王楠, 汤文军. 大位阻膦配体在钯催化芳基烷基交叉偶联反应中的应用★[J]. 有机化学, 2025, 45(9): 3351-3360. |
| [7] | 李金霞, 邓远程, 李嘉瑜, 郭益豪, 王广凤, 段阿冰, 瞿双林. 钯催化硅环开环/交叉偶联反应机理研究[J]. 有机化学, 2025, 45(8): 2938-2944. |
| [8] | 王静, 陈宇讯, 姜静静. 新型茚并芴-5,7-二酮衍生物的合成与性质研究[J]. 有机化学, 2025, 45(7): 2444-2450. |
| [9] | 王超, 陈洪平, 米明众, 李澳文, 齐燕, 刘永军. 锰催化的有机合成偶联反应进展[J]. 有机化学, 2025, 45(7): 2326-2334. |
| [10] | 杜一鸣, 贾均松, 李玉龙, 舒伟. 手性α-芳基酮的催化合成研究进展[J]. 有机化学, 2025, 45(6): 1838-1870. |
| [11] | 苏雷, 杨熙, 闫捷, 蒋元力, 陈丽娟, 郑庆舒, 刘家旺. 不对称羰基化偶联反应研究进展[J]. 有机化学, 2025, 45(6): 2007-2047. |
| [12] | 张艮红, 余若曦, 陈跃刚. 光/电促进醇及其衍生物C—O键活化构筑C(sp2)—C(sp3)键研究进展[J]. 有机化学, 2025, 45(5): 1548-1568. |
| [13] | 李赛洛, 马大为. 碘化亚铜/4-羟基吡啶酰肼催化的(杂)芳基氯代物和苯基亚磺酸钠的偶联反应[J]. 有机化学, 2025, 45(4): 1276-1282. |
| [14] | 王晓琴, 许盛, 平媛媛, 孔望清. 光/镍协同催化实现C(sp3)—H键选择性官能团化[J]. 有机化学, 2025, 45(2): 383-422. |
| [15] | 王浩洋, 成琳, 曾星, 韩利民, 杜玉英, 竺宁. 功能化离子液体[BMim]OH催化α-卤代物与亚磺酸钠盐合成β-酮砜及砜类化合物[J]. 有机化学, 2025, 45(1): 246-255. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||