Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (11): 3640-3657.DOI: 10.6023/cjoc202205001 Previous Articles Next Articles
REVIEWS
收稿日期:2022-05-01
修回日期:2022-06-13
发布日期:2022-06-29
通讯作者:
杨武林
基金资助:
Hui Yan, Man Zhang, Lin Li, Teng Hu, Wulin Yang( )
)
Received:2022-05-01
Revised:2022-06-13
Published:2022-06-29
Contact:
Wulin Yang
Supported by:Share
Hui Yan, Man Zhang, Lin Li, Teng Hu, Wulin Yang. Advances in the Catalytic Asymmetric Synthesis of Chiral Spiroketals[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3640-3657.
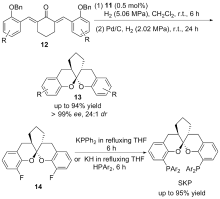
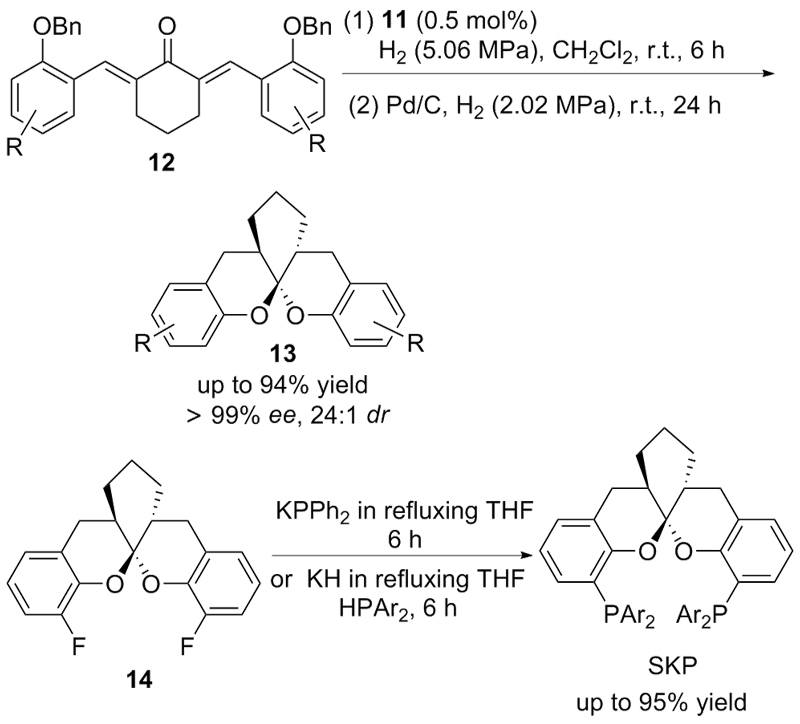
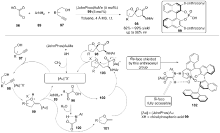

| [1] |
(a) Zhu, S.-F.; Zhou, Q.-L. In Privileged Chiral Ligands and Catalysts, Ed.: Zhou, Q.-L., Wiley-VCH, Weinheim, 2011, pp. 137-170.
pmid: 21975423 |
|
(b) Ding, K.; Han, Z.; Wang, Z. Chem. Asian J. 2009, 4, 32.
doi: 10.1002/asia.200800192 pmid: 21975423 |
|
|
(c) Ramon, R. Chem. Soc. Rev. 2012, 41, 1060.
doi: 10.1039/c1cs15156h pmid: 21975423 |
|
| [2] |
(a) Perron, F.; Albizati, K. F. Chem. Rev. 1989, 89, 1617.
doi: 10.1021/cr00097a015 |
|
(b) Wilsdorf, M.; Reissig, H. U. Angew. Chem., Int. Ed. 2012, 51, 9486.
doi: 10.1002/anie.201203847 |
|
| [3] |
(a) Aho, J. E.; Pihko, P. M.; Rissa, T. K. Chem. Rev. 2005, 105, 4406.
doi: 10.1021/cr050559n pmid: 31478048 |
|
(b) Sperry, J.; Wilson, Z. E.; Rathwell, D. C. K.; Brimble, M. A. Nat. Prod. Rep. 2010, 27, 1117.
doi: 10.1039/b911514p pmid: 31478048 |
|
|
(c) Atkinson, D. J.; Brimble, M. A. Nat. Prod. Rep. 2015, 32, 811.
doi: 10.1039/c4np00153b pmid: 31478048 |
|
|
(d) Zhang, F.-M.; Zhang, S.-Y.; Tu, Y.-Q. Nat. Prod. Rep. 2018, 35, 75.
doi: 10.1039/C7NP00043J pmid: 31478048 |
|
|
(e) Gillard, R. M.; Brimble, M. A. Org. Biomol. Chem. 2019, 17, 8272.
doi: 10.1039/c9ob01598a pmid: 31478048 |
|
| [4] |
Liu, W.-Z.; Ma, L.-Y.; Liu, D.-S.; Huang, Y.-L.; Wang, C.-H.; Shi, S.-S.; Pan, X.-H.; Song, X.-D.; Zhu, R.-X. Org. Lett. 2014, 16, 90.
doi: 10.1021/ol403076s |
| [5] |
Fujimoto, H.; Nozawa, M.; Okuyama, E.; Ishibashi, M. Chem. Pharm. Bull. 2002, 50, 330.
doi: 10.1248/cpb.50.330 |
| [6] |
Li, J.; Li, L.; Si, Y.; Jiang, X.; Guo, L.; Che, Y. Org. Lett. 2011, 13, 2670.
doi: 10.1021/ol200770k pmid: 21495643 |
| [7] |
Stierle, A. A.; Stierle, D. B.; Kelly, K. J. Org. Chem. 2006, 71, 5357;
doi: 10.1021/jo060018d |
| [8] |
Zhuravleva, O. I.; Sobolevskaya, M. P.; Afiyatullov, S. S.; Kirichuk, N. N.; Denisenko, V. A.; Dmitrenok, P. S.; Yurchenko, E. A.; Dyshlovoy, S. A. Mar. Drugs 2014, 12, 5930.
doi: 10.3390/md12125930 pmid: 25501795 |
| [9] |
Namikoshi, M.; Kobayashi, H.; Yoshimoto, T.; Meguro, S. Chem. Lett. 2000, 29, 308.
doi: 10.1246/cl.2000.308 |
| [10] |
(a) Twiner, M. J.; Doucette, G. J.; Pang, Y.; Fang, C.; Forsyth, C. J.; Miles, C. O. Mar. Drugs 2016, 14, 207.
doi: 10.3390/md14110207 |
|
(b) Fu, L.-L.; Zhao, X.-Y.; Ji, L.-D.; Xu, J. Toxicon 2019, 160, 1.
doi: 10.1016/j.toxicon.2018.12.007 |
|
| [11] |
Reddy, C. R.; Srikanth, B.; Dilipkumar, U.; Rao, K. M. V.; Jagadeesh, B. Eur. J. Org. Chem. 2013, 525.
|
| [12] |
Trost, B. M.; Weiss, A. H. Angew. Chem., Int. Ed. 2007, 46, 7664.
doi: 10.1002/anie.200702637 |
| [13] |
Uckun, F. M.; Mao, C.; Vassilev, A. O.; Huang, H.; Jan, S.-T. Bioorg. Med. Chem. Lett. 2000, 10, 541.
pmid: 10741549 |
| [14] |
Scheepstra, M.; Andrei, S. A.; Unver, M. Y.; Hirsch, A. K. H.; Leysen, S.; Ottmann, C.; Brunsveld, L.; Milroy, L.-G. Angew. Chem., Int. Ed. 2017, 56, 5480.
doi: 10.1002/anie.201612504 |
| [15] |
(a) Wang, X.; Ding, K. Chin. J. Chem. 2018, 36, 899.
doi: 10.1002/cjoc.201800247 |
|
(b) Wang, X.; Han, Z.; Wang, Z.; Ding, K. Acc. Chem. Res. 2021, 54, 668.
doi: 10.1021/acs.accounts.0c00697 |
|
|
(c) Li, J.; Chen, G.; Wang, Z.; Zhang, R.; Zhang, X.; Ding, K. Chem. Sci. 2011, 2, 1141.
doi: 10.1039/c0sc00607f |
|
| [16] |
(a) Argüelles, A. J.; Sun, S.; Budaitis, B. G.; Nagorny, P. Angew. Chem., Int. Ed. 2018, 57, 5325.
doi: 10.1002/anie.201713304 pmid: 30207727 |
|
(b) Huang, J.; Hong, M.; Wang, C.-C.; Kramer, S.; Lin, G.-Q.; Sun, X.-W. J. Org. Chem. 2018, 83, 12838.
doi: 10.1021/acs.joc.8b01693 pmid: 30207727 |
|
| [17] |
(a) Rizzacasa, M. A.; Pollex, A. Org. Biomol. Chem. 2009, 7, 1053.
doi: 10.1039/b819966n pmid: 19262920 |
|
(b) Raju, B. R.; Saikia, A. K. Molecules 2008, 13, 1942.
doi: 10.3390/molecules13081942 pmid: 19262920 |
|
|
(c) Sperry, J.; Liu, Y.-C.; Brimble, M. A. Org. Biomol. Chem. 2010, 8, 29.
pmid: 19262920 |
|
|
(d) Palmes, J. A.; Aponick, A. Synthesis 2012, 44, 3699.
doi: 10.1055/s-0032-1317489 pmid: 19262920 |
|
|
(e) Quach, R.; Chorley, D. F.; Brimble, M. A. Org. Biomol. Chem. 2014, 12, 7423.
doi: 10.1039/C4OB01325E pmid: 19262920 |
|
|
(f) Quach, R.; Furkert, D. P.; Brimble, M. A. Org. Biomol. Chem. 2017, 15, 3098.
doi: 10.1039/C7OB00496F pmid: 19262920 |
|
| [18] |
Čorić, I.; List, B. Nature 2012, 483, 315.
doi: 10.1038/nature10932 |
| [19] |
(a) Sun, Z.; Winschel, G. A.; Borovika, A.; Nagorny, P. J. Am. Chem. Soc. 2012, 134, 8074.
doi: 10.1021/ja302704m pmid: 26641317 |
|
(b) Khomutnyk, Y. Y.; Argüelles, A. J.; Winschel, G. A.; Sun, Z.; Zimmerman, P. M.; Nagorny, P. J. Am. Chem. Soc. 2016, 138, 444.
doi: 10.1021/jacs.5b12528 pmid: 26641317 |
|
| [20] |
Wang, X.; Han, Z.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2012, 51, 936;
doi: 10.1002/anie.201106488 |
| [21] |
Wang, X.; Guo, P.; Wang, X.; Wang, Z.; Ding, K. Adv. Synth. Catal. 2013, 355, 2900.
doi: 10.1002/adsc.201300380 |
| [22] |
Liu, N.; Zhu, W.; Yao, J.; Yin, L.; Lu, T.; Dou, X. ACS Catal. 2020, 10, 2596.
doi: 10.1021/acscatal.9b05577 |
| [23] |
Han, X.; Floreancig, P. E. Angew. Chem., Int. Ed. 2014, 53, 11075.
doi: 10.1002/anie.201406819 |
| [24] |
Rexit, A. A.; Mailikezati, M. Tetrahedron Lett. 2015, 56, 2651.
doi: 10.1016/j.tetlet.2015.03.007 |
| [25] |
Yoneda, N.; Fukata, Y.; Asano, K.; Matsubara, S. Angew. Chem., Int. Ed. 2015, 54, 15497.
doi: 10.1002/anie.201508405 |
| [26] |
Xue, J.; Zhang, H.; Tian, T.; Yin, K.; Liu, D.; Jiang, X.; Li, Y.; Jin, X.; Yao, X. Adv. Synth. Catal. 2016, 358, 370.
doi: 10.1002/adsc.201500390 |
| [27] |
Hamilton, J. Y.; Rössler, S. L.; Carreira, E. M. J. Am. Chem. Soc. 2017, 139, 8082.
doi: 10.1021/jacs.7b02856 pmid: 28598614 |
| [28] |
Rössler, S. L.; Schreib, B. S.; Ginterseder, M.; Hamilton, J. Y.; Carreira, E. M. Org. Lett. 2017, 19, 5533.
doi: 10.1021/acs.orglett.7b02620 pmid: 28968123 |
| [29] |
Midya, A.; Maity, S.; Ghorai, P. Chem. Eur. J. 2017, 23, 11216.
doi: 10.1002/chem.201701291 |
| [30] |
Roy, T. K.; Gorad, S. S.; Ghorai, P. Org. Lett. 2022, 24, 1889.
doi: 10.1021/acs.orglett.2c00074 |
| [31] |
Reddy, R. R.; Panda, S.; Ghorai, P. J. Org. Chem. 2019, 84, 5357.
doi: 10.1021/acs.joc.9b00371 |
| [32] |
Zheng, T.; Wang, X.; Ng, W.-H.; Tse, Y.-L. S.; Yeung, Y.-Y. Nat. Catal. 2020, 3, 993.
doi: 10.1038/s41929-020-00530-9 |
| [33] |
Xu, S.; Huang, A.; Yang, Y.; Wang, Y.; Zhang, M.; Sun, Z.; Zhao, M.; Wei, Y.; Li, G.; Hong, L. Org. Lett. 2022, 24, 2978.
doi: 10.1021/acs.orglett.2c00845 |
| [34] |
Hilby, K. M.; Denmark, S. E. J. Org. Chem. 2021, 86, 14250.
doi: 10.1021/acs.joc.1c02271 |
| [35] |
Audrain, H.; Thorhauge, J.; Hazell, R. G.; Jørgensen, K.-A. J. Org. Chem. 2000, 65, 4487.
pmid: 10959849 |
| [36] |
Yu, S.; Gao, L.; Yan, Y.; Yin, Z.; Shang, Y. Chin. J. Org. Chem. 2021, 41, 582. (in Chinese)
doi: 10.6023/cjoc202006050 |
|
( 余述燕, 高丽宏, 闫溢哲, 尹志刚, 商永嘉, 有机化学 2021, 41, 582.)
doi: 10.6023/cjoc202006050 |
|
| [37] |
Wu, H.; He, Y.-P.; Gong, L.-Z. Org. Lett. 2013, 15, 460.
doi: 10.1021/ol303188u |
| [38] |
Cala, L.; Mendoza, A.; Fañanás, F. J.; Rodríguez, F. Chem. Commun. 2013, 49, 2715.
doi: 10.1039/c3cc00118k |
| [39] |
(a) Wang, X.; Dong, S.; Yao, Z.; Feng, L.; Daka, P.; Wang, H.; Xu, Z. Org. Lett. 2014, 16, 22.
doi: 10.1021/ol4033286 pmid: 28474897 |
|
(b) Liang, M.; Zhang, S.; Jia, J.; Tung, C.-H.; Wang, J.; Xu, Z. Org. Lett. 2017, 19, 2526.
doi: 10.1021/acs.orglett.7b00804 pmid: 28474897 |
|
|
(c) Teng, Q.; Qi, J.; Zhou, L.; Xu, Z.; Tung, C.-H. Org. Chem. Front. 2018, 5, 990.
doi: 10.1039/C7QO01005B pmid: 28474897 |
|
|
(d) Mao, W.; Lin, S.; Zhang, L.; Lu, H.; Jia, J.; Xu, Z. Org. Chem. Front. 2020, 7, 856.
doi: 10.1039/D0QO00022A pmid: 28474897 |
|
| [40] |
Li, J.; Lin, L.; Hu, B.; Lian, X.; Wang, G.; Liu, X.; Feng, X. Angew. Chem., Int. Ed. 2016, 55, 6075.
doi: 10.1002/anie.201601701 |
| [41] |
Gong, J.; Wan, Q.; Kang, Q. Adv. Synth. Catal. 2018, 360, 4031.
doi: 10.1002/adsc.201800492 |
| [42] |
(a) Yang, W.-L.; Shang, X.-Y.; Luo, X.; Deng, W.-P. Angew. Chem., Int. Ed. 2022, 61, e202203661.
|
|
(b) Yuen, D.-Y.; Yang, S.-H.; Brimble, M. A. Angew. Chem., nt. Ed. 2011, 50, 8350.
|
|
| [43] |
Yang, W.-L.; Wang, Y.-L.; Li, W.; Gu, B.-M.; Wang, S.-W.; Luo, X.; Tian, B.-X.; Deng, W.-P. ACS Catal. 2021, 11, 12557.
doi: 10.1021/acscatal.1c03711 |
| [44] |
Chen, J.-R.; Hu, X.-Q.; Xiao, W.-J. Angew. Chem., Int. Ed. 2014, 53, 4038.
doi: 10.1002/anie.201400018 |
| [45] |
(a) Wang, D.; Liu, S.; Lan, X.-C.; Paniagua, A.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. Adv. Synth. Catal. 2017, 359, 3186.
doi: 10.1002/adsc.201700543 |
|
(b) Liu, S.; Chen, K.; Lan, X.-C.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. Chem. Commun. 2017, 53, 10692.
doi: 10.1039/C7CC05563C |
|
|
(c) Liu, S.; Lan, X.-C.; Chen, K.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. Org. Lett. 2017, 19, 3831.
doi: 10.1021/acs.orglett.7b01705 |
|
|
(d) Qiu, J.-K.; Hao, W.-J.; Li, G.; Jiang, B. Adv. Synth. Catal. 2018, 360, 1182.
doi: 10.1002/adsc.201701149 |
|
|
(e) Ji, C.-L.; Pan, Y.; Geng, F.-Z.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Org. Chem. Front. 2019, 6, 474.
doi: 10.1039/C8QO01277F |
|
| [46] |
Ge, S.; Cao, W.; Kang, T.; Hu, B.; Zhang, H.; Su, Z.; Liu, X.; Feng, X. Angew. Chem., Int. Ed. 2019, 58, 4017.
doi: 10.1002/anie.201812842 |
| [47] |
Dong, K.; Gurung, R.; Xu, X.; Doyle, M. P. Org. Lett. 2021, 23, 3955.
doi: 10.1021/acs.orglett.1c01113 |
| [1] | Shuang Yang, Xinqiang Fang. Kinetic Resolutions Enabled by N-Heterocyclic Carbene Catalysis: An Update [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 448-480. |
| [2] | Wanting Chen, Xiongwei Zhong, Jiale Xing, Changshu Wu, Yang Gao. Progress in Asymmetric Catalytic Synthesis of C—N Axis Chiral Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 349-377. |
| [3] | Quanbin Jiang. Progress in Synthesis of Axially Chiral Compounds through aza-Vinylidene o-Quinone Methide Intermediates [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 159-172. |
| [4] | Chun-Xia Cheng, Lu-Ping Wu, Feng Sha, Xin-Yan Wu. Enantioselective Vinylogous Allylic Alkylation of Coumarins with Morita-Baylis-Hillman Carbonates Catalyzed by Chiral Phosphine-Amide [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3188-3195. |
| [5] | Cheng Luo, Yanli Yin, Zhiyong Jiang. Recent Advances in Asymmetric Synthesis of P-Chiral Phosphine Oxides [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1963-1976. |
| [6] | Haiqing Wang, Shuang Yang, Yuchen Zhang, Feng Shi. Advances in Catalytic Asymmetric Reactions Involving o-Hydroxybenzyl Alcohols [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 974-999. |
| [7] | Weidi Cao, Xiaohua Liu. Recent Advances on Catalytic Enantioselective Protonation for Construction of α-Tertiary Carbonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 961-973. |
| [8] | Siqiang Fang, Zanjiao Liu, Tianli Wang. Recent Advances of the Atherton-Todd Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1069-1083. |
| [9] | Xin Kuang, Changhua Ding, Yichen Wu, Peng Wang. Catalytic Enantioselective Preparation of Chiral Allylsilanes [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3367-3387. |
| [10] | Yan Zeng, Fei Ye. Research Progress on New Catalytic Reaction Systems for Asymmetric Synthesis of Silicon-Stereogenic Center Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3388-3413. |
| [11] | Jiayi Zhao, Yicong Ge, Chuan He. Construction of Silicon-Stereogenic Center via Catalytic Asymmetric Si—H/X—H Dehydrogenative Coupling [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3352-3366. |
| [12] | Zengjin Dai, Xumu Zhang, Qin Yin. Advances on Asymmetric Reductive Amination with Ammonium Salts as Amine Sources [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2261-2274. |
| [13] | Hui Li, Liang Yin. Research Progress of Copper-Catalyzed Direct Vinylogous Reactions [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1573-1585. |
| [14] | Mengmeng Xu, Quan Cai. Progress of Catalytic Asymmetric Diels-Alder Reactions of 2-Pyrones [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 698-713. |
| [15] | Yunrong Chen, Wei Liu, Xiaoyu Yang. Recent Advances in Kinetic Resolution of Tertiary Alcohols [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 679-697. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||