Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (8): 2545-2553.DOI: 10.6023/cjoc202403032 Previous Articles Next Articles
ARTICLES
宫清宝a, 吕翔b, 于长江b,*( ), 李婉婉b, 赵全胜b,*(
), 李婉婉b, 赵全胜b,*( ), 焦莉娟b, 郝二红b,*(
), 焦莉娟b, 郝二红b,*( )
)
收稿日期:2024-03-22
修回日期:2024-05-11
发布日期:2024-06-13
基金资助:
Qingbao Gonga, Xiang Lüb, Changjiang Yub( ), Wanwan Lib, Quansheng Zhaob(
), Wanwan Lib, Quansheng Zhaob( ), Lijuan Jiaob, Erhong Haob(
), Lijuan Jiaob, Erhong Haob( )
)
Received:2024-03-22
Revised:2024-05-11
Published:2024-06-13
Contact:
E-mail: Supported by:Share
Qingbao Gong, Xiang Lü, Changjiang Yu, Wanwan Li, Quansheng Zhao, Lijuan Jiao, Erhong Hao. Aggregation-Induced Emission (AIE) Active Fluoroboronated Pyridylhydrazinyl Aldehyde Hydrozone Dyes: Synthesis, Crystal Structure and Optical Properties[J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2545-2553.
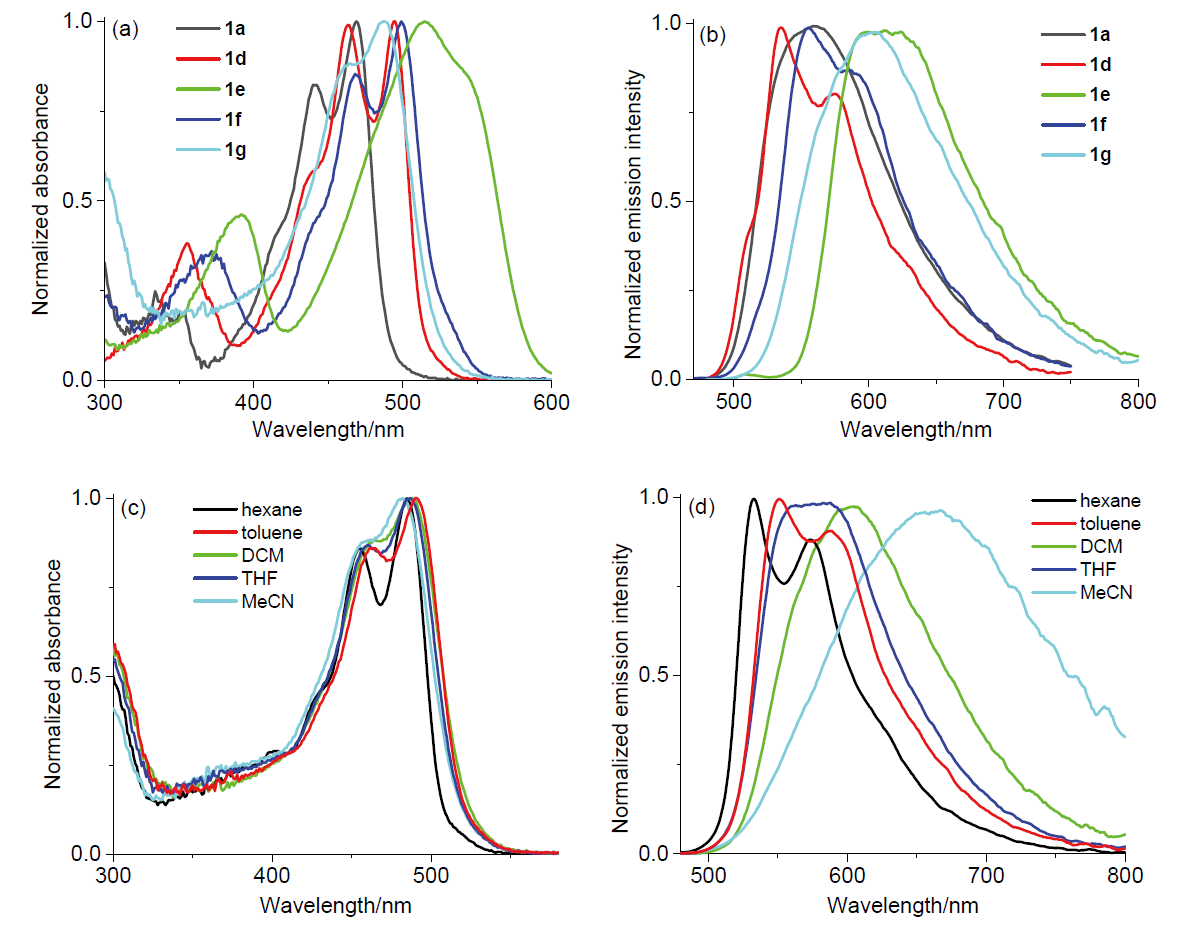
| Dye | Dichloromethane | Solid powder | |||||
|---|---|---|---|---|---|---|---|
| λabsmax/nm | λemmax/nm | εb | Stokes-shift/cm-1 | ϕc | λem/nm (ϕd) | ||
| 1a | 472 | 552 | 32400 | 3100 | 0.02 | 578 (0.10) | |
| 1b | 492 | 533 | 49000 | 1700 | 0.02 | 609 (0.10) | |
| 1c | 469 | 560 | 36300 | 3500 | 0.06 | 566 (0.12) | |
| 1d | 495 | 535 | 44700 | 1500 | 0.01 | 625 (0.05) | |
| 1e | 515 | 613 | 42700 | 3100 | 0.01 | 750 (0.02) | |
| 1f | 499 | 556 | 47900 | 2100 | 0.01 | 608 (0.05) | |
| 1g | 488 | 605 | 46800 | 4000 | 0.02 | 617 (0.06) | |
| Dye | Dichloromethane | Solid powder | |||||
|---|---|---|---|---|---|---|---|
| λabsmax/nm | λemmax/nm | εb | Stokes-shift/cm-1 | ϕc | λem/nm (ϕd) | ||
| 1a | 472 | 552 | 32400 | 3100 | 0.02 | 578 (0.10) | |
| 1b | 492 | 533 | 49000 | 1700 | 0.02 | 609 (0.10) | |
| 1c | 469 | 560 | 36300 | 3500 | 0.06 | 566 (0.12) | |
| 1d | 495 | 535 | 44700 | 1500 | 0.01 | 625 (0.05) | |
| 1e | 515 | 613 | 42700 | 3100 | 0.01 | 750 (0.02) | |
| 1f | 499 | 556 | 47900 | 2100 | 0.01 | 608 (0.05) | |
| 1g | 488 | 605 | 46800 | 4000 | 0.02 | 617 (0.06) | |
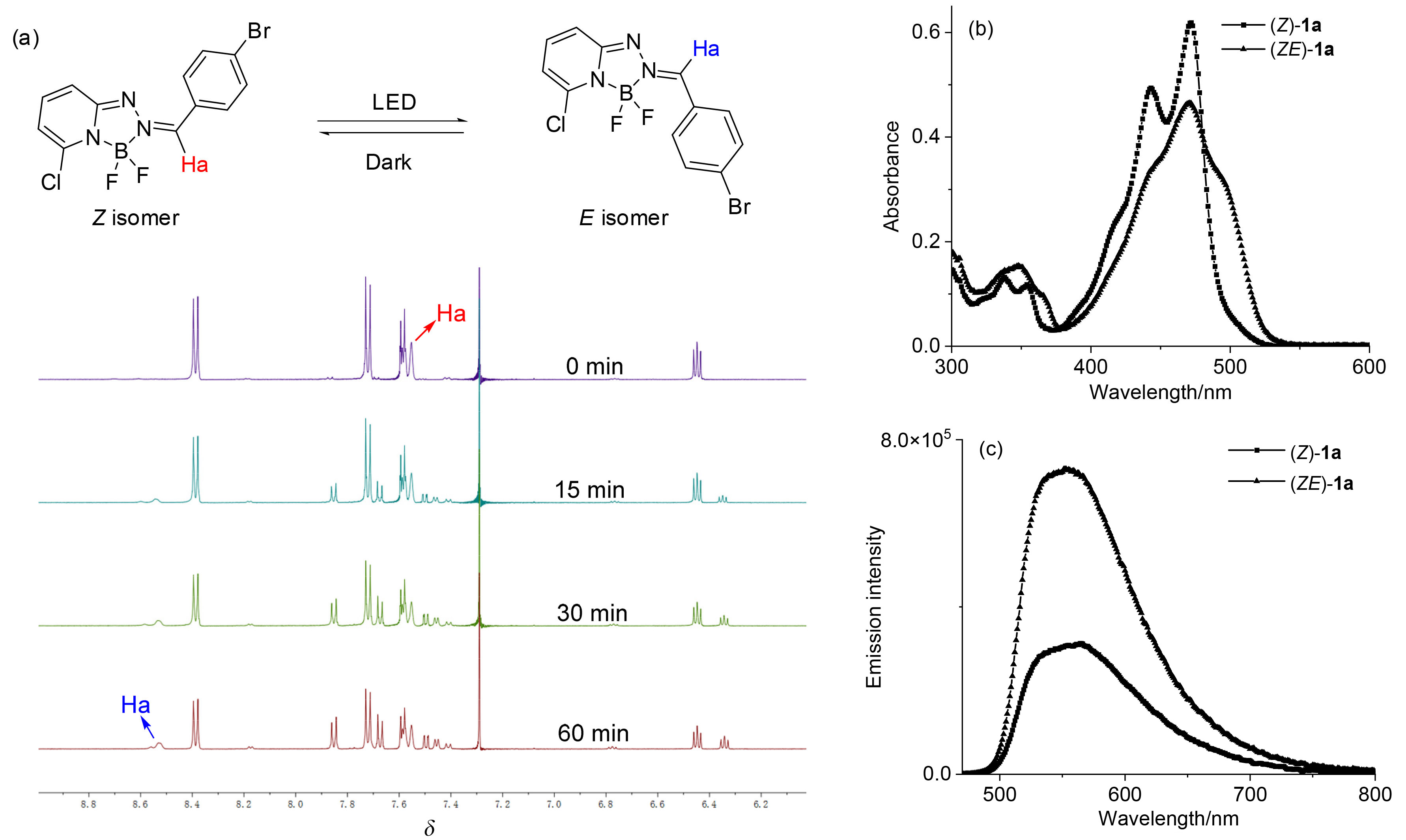
| [1] |
Chen, H.; Han, P.; Qin, A.; Tang, B. Z. Acta Chim. Sinic. 2023, 81, 1420 (in Chinese).
|
|
(徐赫, 韩鹏博, 秦安军, 唐本忠, 化学学报, 2023, 81, 1420.)
|
|
| [2] |
Mei, J.; Leung, N. L. C.; Kwok, R. T. K.; Lam, J. W. Y.; Tang, B. Z. Chem. Rev. 2015, 115, 11718.
doi: 10.1021/acs.chemrev.5b00263 pmid: 26492387 |
| [3] |
Zhu, F.-Y.; Mei, L.-J.; Tian, R.; Li, C.; Xiang, S.-L.; Zhu, M.-Q.; Wang, Y.-L.; Tang, B. Z. Chem. Soc. Rev. 2024, 53, 3350.
|
| [4] |
Chen, M.; Dong, R.; Song, J.; Qi, J.; Zhang, J.; Zhao, Z.; Zhang, W.; Li, Y.; Tang, B. Z. Adv. Healthcare Mate.. 2024, 13, 2303967.
|
| [5] |
Yang, L.-L.; Wang, H.; Zhang, J.; Wu, B.; Li, Q.; Chen, J.-Y.; Tang, A.-L.; Lam, J. W. Y.; Zhao, Z.; Yang, S.; Tang, B. Z. Nat. Commun. 2024, 15, 999.
|
| [6] |
Chen, Y.-J.; Pu, M.-Q.; Wu, L.-T.; Sun, X.-L.; Wan, W.-M. Chin. J. Chem. 2023, 41, 1705.
|
| [7] |
Luo, J.; Xie, Z.; Lam, J. W. Y.; Cheng, L.; Chen, H.; Qiu, C.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 18, 1740.
|
| [8] |
Zhao, Y.; Chen, P.; Li, G.; Niu, Z.; Wang, E. Chin. J. Org. Che.. 2023, 43, 2156 (in Chinese).
|
|
(赵洋, 陈盼盼, 李高楠, 钮智刚, 王恩举, 有机化学, 2023, 43, 2156.)
|
|
| [9] |
Gui, Y.; Chen, K.; Sun, Y.; Tan, Y.; Luo, W.; Zhu, D.; Xiong, Y.; Yan, D.; Wang, D.; Tang, B. Z. Chin. J. Chem. 2023, 41, 1249.
|
| [10] |
Long, R.; Tang, C.; Xu, J.; Li, T.; Tong, C.; Guo, Y.; Shi, S.; Wang, D. Chem. Commun. 2019, 55, 10912.
|
| [11] |
Kim, D.; Lee, U.; Bouffard, J. Kim, Y. Adv. Opt. Mater. 2020, 8, 1902161.
|
| [12] |
Han, G.; Kim, D.; Park, Y.; Bouffard, J.; Kim, Y. Angew. Che.. 2015, 127, 3984.
|
| [13] |
Marsh, A. V.; Cheetham, N. J.; Little, M.; Dyson, M.; White, A. J.; Beavis, P.; Warriner, C. N.; Swain, A. C.; Stavrinou, P. N.; Heeney, M. Angew. Che.. 2018, 130, 10800.
|
| [14] |
Zeng, C.; Hu, P.; Wang, B.; Fang, W.; Zhao, K. Chin. J. Org. Che.. 2023, 43, 3287 (in Chinese).
|
|
(曾崇洋, 胡平, 汪必琴, 方文彦, 赵可清, 有机化学, 2023, 43, 3287.)
|
|
| [15] |
Huang, W.; Zhao, X.; Zhang, S.; Ying, L.; Miao, X.; Deng, W. Dyes Pig.. 2024, 225, 112063.
|
| [16] |
Bismillah, A. N.; Aprahamian, I. Chem. Soc. Re.. 2021, 50, 5631.
|
| [17] |
Mao, Z.; Kim, J. H.; Lee, J.; Xiong, H.; Zhang, F.; Kim, J. S. Coord. Chem. Re.. 2023, 476, 214908.
|
| [18] |
Chen, Z.; Ni, Z.; Chen, X.-Y.; Xu, Y.; Yu, C.; Wang, S.; Wang, X.-Y.; Lu, H. Aggregat. 2024, 5, e498.
|
| [19] |
Ji, C.; Yang, J.; Hu, S.; Mack, J.; Zhang, Y.; Lu, H.; Gai, L. Dyes Pig.. 2023, 220, 111707.
|
| [20] |
Qian, H.; Cousins, M. E.; Horak, E. H.; Wakefield, A.; Liptak, M. D.; Aprahamian, I. Nat. Chem. 2017, 9, 83.
doi: 10.1038/nchem.2612 pmid: 27995926 |
| [21] |
Murali, A. C.; Nayak, P.; Panda, R.; Das, R.; Venkatasubbaiah, K. ACS Appl. Opt. Mate.. 2023, 1, 1033.
|
| [22] |
Tanaka, K.; Gon, M.; Ito, S.; Ochi, J.; Chujo, Y. Coord. Chem. Re.. 2022, 472, 214779.
|
| [23] |
Nakamura, M.; Kanetani, I.; Gon, M.; Tanaka, K. Angew. Chem., Int. Ed. 2024, e202404178.
|
| [24] |
Duan, W.; Liu, Q.; Huo, Y.; Cui, J.; Gong, S.; Liu, Z. Org. Biomol. Chem. 2018, 16, 4977.
|
| [25] |
Yordanov, D.; Smolka, R.; Nakashima, K.; Hirashima, S. I.; Matsushima, Y.; Vala, M.; Krajcovic, J.; Weiter, M.; Miura, T.; Georgiev, A. J. Org. Che.. 2023, 88, 17206.
|
| [26] |
Wang, X.; Wu, Y.; Liu, Q.; Li, Z.; Yan, H.; Ji, C.; Duan, J.; Liu, Z. Chem. Commu.. 2015, 51, 784.
|
| [27] |
Wang, X.; Liu, Q.; Yan, H.; Liu, Z.; Yao, M.; Zhang, Q.; Gong, S.; He, W. Chem. Commun. 2015, 51, 7497.
|
| [28] |
Liu, Q.; Wang, X.; Yan, H.; Wu, Y.; Li, Z.; Gong, S.; Liu, P.; Liu, Z. J. Mater. Chem. C 2015, 3, 2953.
|
| [29] |
Wang, H.; Zhang, C.; Jiang, Z.; Xu, L.; Liu, Z. Dalton. Trans. 2023, 52, 1393.
|
| [30] |
Ni, J.-S.; Zhao, Z.; Liu, H.; Liu, J.; Jiang, M.; Chen, Y.; Kwok, R. T. K.; Lam, J. W. Y.; Peng, Q.; Tang, B. Z. Mater. Chem. Fron.. 2018, 2, 1498
|
| [31] |
Yu, C.; Hao, E.; Fang, X.; Wu, Q.; Wang, L.; Li, J.; Xu, L.; Jiao, L.; Wong, W.-Y. J. Mater. Chem. C 2019, 7, 3269.
|
| [32] |
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
|
| [33] |
Sheldrick, G. M. Acta Crystallogr. 2015, A71, 3.
|
| [34] |
Sheldrick, G. M. Acta Crystallogr. 2008, A64, 112.
|
| [35] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian,Inc., Wallingford, C., 2013.
|
| [1] | Ziran Tang, Hao Sun, Liangliang Zhu. Research Progress of Photoresponsive Photoluminescent Materials with Aggregation-Induced Emission Characteristics [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2393-2412. |
| [2] | Jie Zhang, Nan Li, Na Zhao. Recent Progress of Aggregation-Induced Emission Molecule Nanozyme Composites [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2469-2478. |
| [3] | Yizhuo Shen, Kangwei Luo, Qingyang Xu, Jianyu Zhang, JingZhi Sun, Haoke Zhang, BenZhong Tang. Weak Interaction-Based Organic Luminescent Materials [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2453-2468. |
| [4] | Junchu He, Junqi Wu, Jianghui Wang, Jingwen Xu, BenZhong Tang, Zujin Zhao. Blue Aggregation-Induced Delayed Fluorescence Materials with 5,10-Dihydrodibenzo[b,e][1,4]azasiline as Donor [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2513-2522. |
| [5] | Zhixin Xie, Shaoling Li, Wei Liu, Kai Yan, Tao Jiang, Yiwei Liu, Md. Monarul Islam, Xing Feng. An Efficient Approach to Narrow the Emission Band of Pyrene-Based Emitters [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2504-2512. |
| [6] | Zixiang Meng, Xiumei Tian, Tianfu Zhang. Recent Progress of Aggregation-Induced Emission Materials in Tumor Phototherapy [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2441-2452. |
| [7] | Weigeng Huang, Yiting Gao, Yan Sun, Dingyuan Yan, Dong Wang, BenZhong Tang. Aggregation-Induced Emission Materials for Tumor Phototheranostics [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2413-2424. |
| [8] | Yuanhao Wang, Yukai Sun, Yuhang Liu, Zhaoming Zhang, Xuzhou Yan. Construction and Properties of Flexible Light-Emitting Materials Based on Tetraphenylethylene [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2538-2544. |
| [9] | Hanyu Jia, Yuewen Yu, Guangxue Feng, BenZhong Tang. Construction of Type I Aggregation-Induced Emission Photosensitizers for Photodynamic Therapy via Photoinduced Electron Transfer Mechanism [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2530-2537. |
| [10] | Kaihang Huang, Li Yin, Qingyun Jiang, Qian Wang, Guang Shi, Bingjia Xu. Efficient Thermally Activated Delayed Fluorescence Materials with Aggregation-Induced Emission for Lipid Droplet Imaging [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2479-2486. |
| [11] | Yujie Yang, Wei Cao, Jikai Yu, Zhixia Zhang, Li Xu, Hua Wang. Synthesis of Donor-Acceptor (D-A) Typed Phenylcyclooctatetrathiophenes and Their Performances on Aggregation Induced Emission and High Pressure Luminescence [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2495-2503. |
| [12] | Xiaolong Su, Jianpeng Li, Mengxin Liu, Li Zou, Desuo Yang, Haitao Feng. Synthesis of Tetraphenylethylene Based Amides for Detection of Copper(II) Ion with High Sensitivity and Selectivity [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2581-2587. |
| [13] | Haosen Chang, Liming Yang, Guan Wang, Xinggui Gu. Synthesis of Anthraquinone Derivate with Aggregation-Induced Emission Characteristic for Fingerprint Development [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2571-2580. |
| [14] | Yan Ou, Lin Lan, Zhengxiong Wang, Zhiming Wang, BenZhong Tang. Preparation of Aggregation-Induced Emission Nucleic Acid Probes and Study of Their Nucleic Acid Sensing Principles [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2554-2562. |
| [15] | Yiping Sun, Demao Chen, Ling He, Zuli Wang. Na2S2O8 Mediated C—H Amination of Imidazo[1,2-α]pyridines with Heteroaromatic Amines under Metal-Free Conditions [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1667-1674. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||