有机化学 ›› 2023, Vol. 43 ›› Issue (6): 1963-1976.DOI: 10.6023/cjoc202212024 上一篇 下一篇
综述与进展
收稿日期:2022-12-17
修回日期:2023-01-09
发布日期:2023-01-18
基金资助:
Cheng Luoa,b, Yanli Yina,b,*( ), Zhiyong Jiangb,*(
), Zhiyong Jiangb,*( )
)
Received:2022-12-17
Revised:2023-01-09
Published:2023-01-18
Contact:
E-mail: Supported by:文章分享

手性有机膦化合物在配体、催化剂以及生物活性分子等领域有着广泛的应用. 因此, 通过不对称合成高效构建含P-手性核心骨架化合物吸引了众多化学家的深入研究, 为丰富手性有机膦配体库以及潜在药用活性分子已起到了至关重要的促进作用. 由于五价膦氧化物相对于三价有机膦化合物更稳定且可通过一步还原即可得到三价有机膦化合物, 针对P-手性五价膦氧化合物的不对称合成也因此尤为受到关注, 并快速发展. 基于此, 并针对已报道的相关综述的具体情况, 总结了近三年来不对称合成P-手性膦氧化物的进展, 依据合成策略的不同, 分为手性助剂协助分离、催化去对称化、催化不对称合成、催化动力学拆分或动态动力学不对称转化以及酶催化五个方面进行阐释与讨论.
罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976.
Cheng Luo, Yanli Yin, Zhiyong Jiang. Recent Advances in Asymmetric Synthesis of P-Chiral Phosphine Oxides[J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1963-1976.
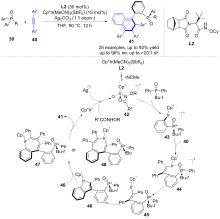
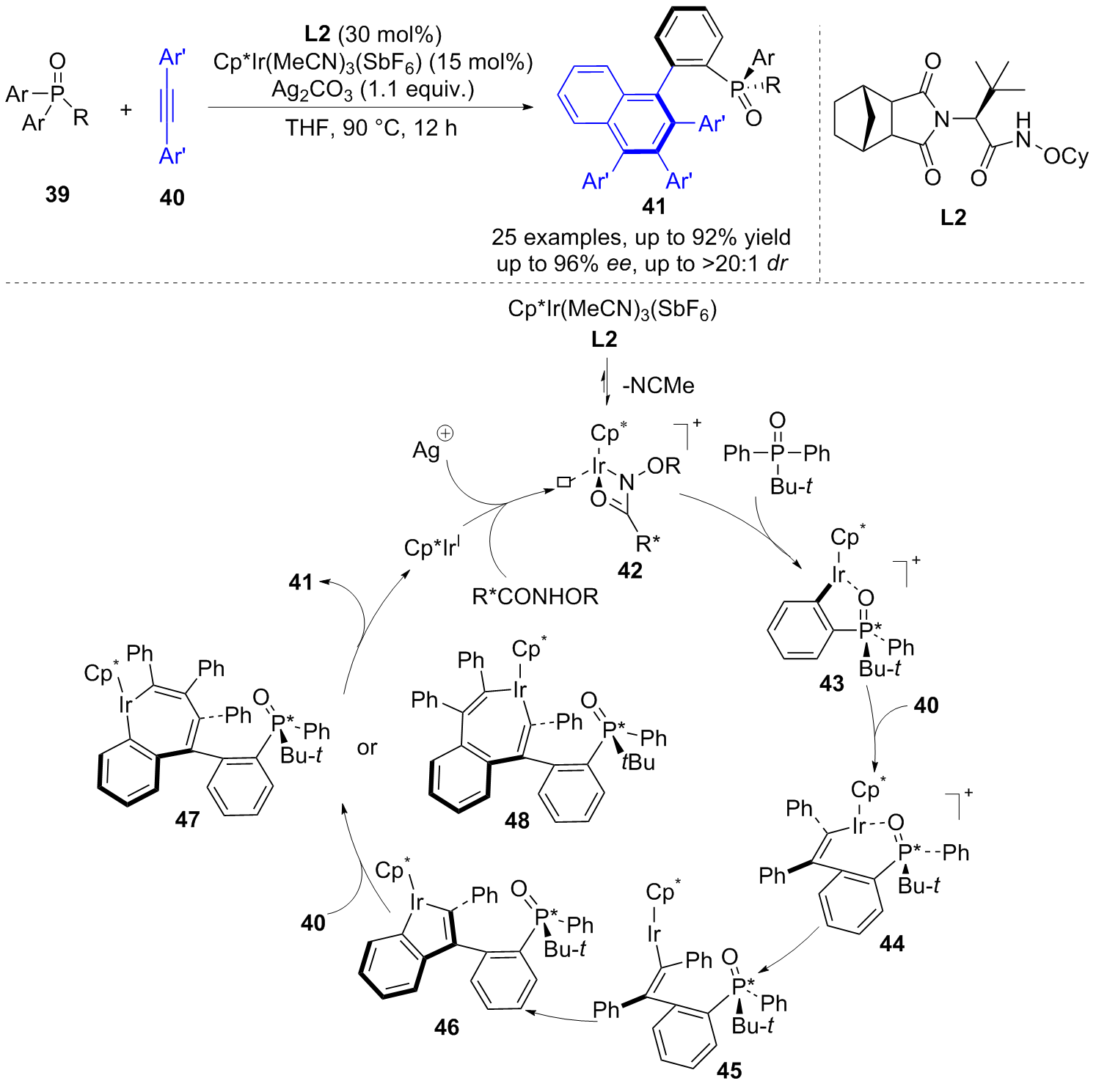
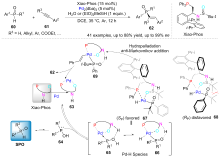
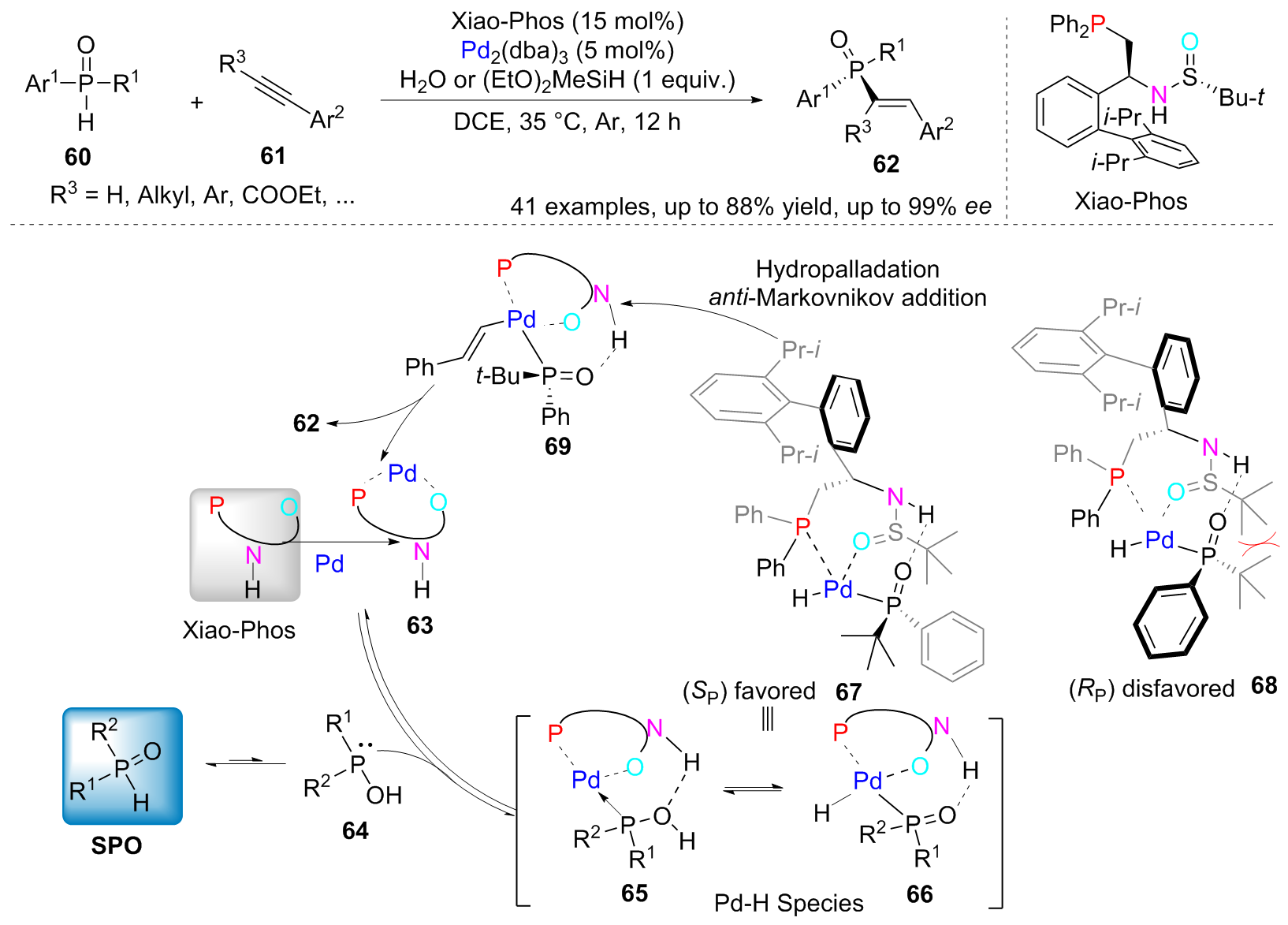
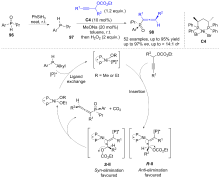

| [1] |
(a) Xie, J. H.; Zhou, Q. L. Acc. Chem. Res. 2008, 41, 581.
doi: 10.1021/ar700137z pmid: 27479243 |
|
(b) Fernández-Pérez, H.; Etayo, P.; Panossian, A.; Vidal-Ferran, A. Chem. Rev. 2011, 111, 2119.
doi: 10.1021/cr100244e pmid: 27479243 |
|
|
(c) Dutartre, M.; Bayardon, J.; Jugé, S. Chem. Soc. Rev. 2016, 45, 5771.
pmid: 27479243 |
|
|
(d) Ye, X. Y.; Peng, L.; Bao, X. Z.; Tan, C.-H.; Wang, H. Green Synth. Catal. 2021, 2, 6.
pmid: 27479243 |
|
| [2] |
Pei, M. Y.; Tian, A. Q.; Yang, Q. Q.; Huang, N. Y.; Wang, L.; Li, D. S. Green Synth. Catal. 2023, DOI: 10.1016/j.gresc.2022.10.011.
doi: 10.1016/j.gresc.2022.10.011 |
| [3] |
Vineyard, B. D.; Knowles, W. S.; Sabacky, M. J.; Bachman, G. L.; Weinkauff, D. J. J. Am. Chem. Soc. 1977, 99, 5946.
doi: 10.1021/ja00460a018 |
| [4] |
Zhu, R. Y.; Liao, K.; Yu, J.; Zhou, J. Acta Chim. Sinica 2020, 78, 193. (in Chinese)
doi: 10.6023/A20010002 |
|
(朱仁义, 廖奎, 余金生, 周剑, 化学学报, 2020, 78, 193.)
doi: 10.6023/A20010002 |
|
| [5] |
Xu, R. H.; Gao, Z. H.; Yu, Y. T.; Tang, Y. H.; Tian, D. S.; Chen, T.; Chen, Y. B.; Xu, G. Q.; Shi, E. X.; Tang, W. J. Chem. Commun. 2021, 57, 3335.
doi: 10.1039/D1CC00646K |
| [6] |
Zhang, Y.; Yuan, J.; Huang, G. L.; Yu, H.; Liu, J. P.; Chen, J.; Meng, S. X.; Zhong, J. J.; Dang, L.; Yu, G. A.; Che, C. M. Chem. Sci. 2022, 13, 6519.
doi: 10.1039/d2sc00036a pmid: 35756532 |
| [7] |
Mondal, A., Thiel, N.O., Dorel, R.; Feringa, B. L. Nat. Catal. 2022, 5, 10.
doi: 10.1038/s41929-021-00697-9 |
| [8] |
(a) Čorić, I.; List, B. Nature 2012, 483, 315.
doi: 10.1038/nature10932 |
|
(b) Xie, Y. W.; List, B. Angew. Chem., Int. Ed. 2017, 56, 4936.
doi: 10.1002/anie.201612149 |
|
|
(c) Yin, Y. L.; Li, Y. Q.; Gonçalves, T. P.; Zhan, Q. Q.; Wang, G. H.; Zhao, X. W.; Qiao, B. K.; Huang, K.-W.; Jiang, Z. Y. J. Am. Chem. Soc. 2020, 142, 19451.
doi: 10.1021/jacs.0c08329 |
|
| [9] |
Guo, R. Z.; Liu, Z. Q.; Zhao, X. D. CCS Chem. 2020, 2, 2617.
|
| [10] |
Huang, Q. H.; Zhou, Q.; Yang, C.; Chen, L.; Cheng, J. P.; Li, X. Chem. Sci. 2021, 12, 4582.
doi: 10.1039/D0SC07008D |
| [11] |
Song, S.; Li, Y.; Ke, Z.; Xu, S. ACS Catal. 2021, 11, 13445.
doi: 10.1021/acscatal.1c03888 |
| [12] |
Zhang, C. W.; Hu, X. Q.; Dai, Y. H.; Yin, P.; Wang, C.; Duan, W. L. ACS Catal. 2022, 12, 193.
doi: 10.1021/acscatal.1c05080 |
| [13] |
Chen, J. H.; Teng, M. Y.; Huang, F. R.; Song, H.; Wang, Z. K.; Zhuang, H. L.; Wu, Y. J.; Wu, X.; Yao, Q. J.; Shi, B. F. Angew. Chem., Int. Ed. 2022, 61, e202210106.
|
| [14] |
Yang, Z.; Gu, X.; Han, L. B.; Wang, J. Chem. Sci. 2020, 11, 7451.
doi: 10.1039/D0SC01049A |
| [15] |
Dai, Q.; Liu, L.; Qian, Y.; Li, W.; Zhang, J. Angew. Chem., Int. Ed. 2020, 59, 20645.
doi: 10.1002/anie.v59.46 |
| [16] |
Zhang, Q.; Liu, X. T.; Wu, Y.; Zhang, Q. W. Org. Lett. 2021, 23, 8683.
doi: 10.1021/acs.orglett.1c02986 pmid: 34734721 |
| [17] |
Cai, W. Q.; Wei, Q.; Zhang, Q. W. Org. Lett. 2022, 24, 1258.
doi: 10.1021/acs.orglett.2c00209 |
| [18] |
Wu, Z. H.; Cheng, A. Q.; Yuan, M.; Zhao, Y. X.; Yang, H. L.; Wei, L. H.; Wang, H. Y.; Wang, T.; Zhang, Z. T.; Duan, W. L. Angew. Chem., Int. Ed. 2021, 60, 27241.
doi: 10.1002/anie.v60.52 |
| [19] |
Toda, Y.; Pink, M.; Johnston, J. N. J. Am. Chem. Soc. 2014, 136, 14734.
doi: 10.1021/ja5088584 |
| [20] |
Zhang, Y. Q.; Han, X. Y.; Wu, Y.; Qi, P. J.; Zhang, Q.; Zhang, Q. W. Chem. Sci. 2022, 13, 4095.
doi: 10.1039/D2SC00091A |
| [21] |
Wang, W. H., Wu, Y., Wang, H. T.; Qi, P. J.; Lan, W. J., Zhang, Q. W. Nat. Synth. 2022, 1, 738.
doi: 10.1038/s44160-022-00123-3 |
| [22] |
Qiu, H. L.; Dai, Q.; He, J. F.; Li, W. B.; Zhang, J. Chem. Sci. 2020, 11, 9983.
doi: 10.1039/D0SC04041J |
| [23] |
Dai, Q.; Liu, L.; Zhang, J. Angew. Chem., Int. Ed. 2021, 60, 27247.
doi: 10.1002/anie.v60.52 |
| [24] |
Dai, Q.; Li, W.; Li, Z.; Zhang, J. J. Am. Chem. Soc. 2019, 141, 20556.
doi: 10.1021/jacs.9b11938 |
| [25] |
Li, Y. L.; Jin, X.; Liu, P.; Zhang, H. J.; Yu, X. L.; Liu, Y. J.; Liu, B. H.; Yang, W. Q. Angew. Chem., Int. Ed. 2022, 61, e202117093.
|
| [26] |
Bigley, A. N.; Narindoshvili, T.; Raushel, F. M. Biochemistry 2020, 59, 3038.
doi: 10.1021/acs.biochem.0c00591 |
| [27] |
Madalińska, L.; Kiełbasiński, P.; Kwiatkowska, M. Catalysts 2022, 12, 171.
doi: 10.3390/catal12020171 |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [4] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [5] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [6] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [7] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [8] | 胡慧娟, 闫巧丽, 卢晓刚, 杨启帆, 裴承新, 王红梅, 高润利. 猪胰脂肪酶催化外消旋P-手性α-羟基磷酸酯类化合物的动力学拆分[J]. 有机化学, 2023, 43(8): 2815-2825. |
| [9] | 褚杨杨, 韩召斌, 丁奎岭. 动力学拆分在过渡金属催化的不对称(转移)氢化中的应用研究[J]. 有机化学, 2023, 43(6): 1934-1951. |
| [10] | 宋亭谕, 李冉, 黄利华, 贾世琨, 梅光建. N—N单键阻转异构体的催化不对称合成[J]. 有机化学, 2023, 43(6): 1977-1990. |
| [11] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [12] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [13] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [14] | 蒙玲, 汪君. 硫代黄烷酮类衍生物的合成研究进展[J]. 有机化学, 2023, 43(3): 873-891. |
| [15] | 陈宇亮, 贺凤开, 王思云, 贾鼎成, 刘亚群, 黄毅勇. 手性磷酸催化α-全碳季碳醛的不对称烯丙基化动力学拆分[J]. 有机化学, 2023, 43(12): 4294-4302. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||