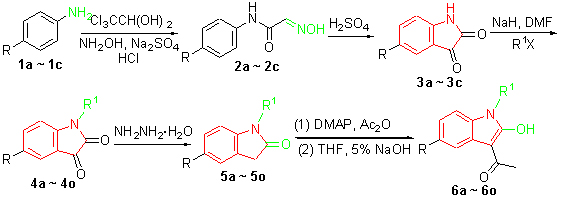
| [1] Jun, Q. R.; Ning, H.; Hui, X.; Liu, M. Y.; Min, L.; Yong, T. Z. Bioorg. Med. Chem. Lett. 2010, 20, 3534.[2] Pais, G. C. G.; Zhang, X.; Marchand, C.; Neamati, N.; Cowansage, K.; Svarovskaia, E. S.; Pathak, V. K.; Tang, Y.; Nicklaus, M.; Pommier, Y.; Burke, Jr. T. R. J. Med. Chem. 2002, 45, 3184.[3] Ferro, S.; Barreca, M. L.; Luca, L. D.; Rao, A.; Monforte, A. M.; Debyser, Z.; Witrouw, M.; Chimirri, A. Arch. Pharm. Chem. Life Sci. 2007, 340, 292.[4] Abdel-gawad, H.; Mohamed, H. A.; Dawood, K. M.; Badria, F. A.-R. Chem. Pharm. Bull. 2010, 58, 1529.[5] Sriniva, P.; Raghavan, S. A. V.; Jagadeeh-bababu, R.; Gupta, C. N. V. H. B.; Sridhar, N.; Veeranjaneyulu, A.; Parimoo, P. Pharmacol. Commun. 1999, 5, 95.[6] Giraud, F.; Alves, G.; Debiton, E.; Nauton, L.; Thery, V.; Durieu, E.; Ferandin, Y.; Lozach, O.; Meijer, L.; Anizon, F.; Pereira, E.; Moreau, P. J. Med. Chem. 2011, 54, 4474.[7] Chennamaneni, S.; Zhong, B.; Lama, R.; Su, B. Eur. J. Med. Chem. 2012, 56, 17.[8] Bruel, A.; Logé, C.; Tauzia, M. L.; Ravache, M.; Guevel, R.; Guillouzo, C.; Lohier, J. F.; Oliveira, Santos. J. S.; Lozach, O.; Meijer, L.; Ruchaud, S.; Bénédetti, H.; Robert, J. M. Eur. J. Med. Chem. 2012, 57, 225.[9] Martel-Frachet, V.; Kadri, M.; Boumendjel, A.; Ronot, X. Bioorg. Med. Chem. 2011, 19, 6143.[10] Kumar, D.; Kumar, N. M.; Sundaree, S.; Johnson, E. O.; Shah, K. Eur. J. Med. Chem. 2010, 45, 1244.[11] Subba Reddy, B. V. S.; Rajeswari, N.; Sarangapani, M.; Reddy, G. R.; Msdan, C.; Kumar, K. P.; Rao, M. S. Bioorg. Med. Chem. Lett. 2011, 21, 6510.[12] Subba, R. B. V.; Rajeswari, N.; Sarangapani, M.; Prashanthi, Y.; Ganji, R. J.; Addlagatta, A. Bioorg. Med. Chem. Lett. 2012, 22, 2460.[13] Jørgensen, M.; Jørgensen, P. N.; Christoffersen, C. T.; Jensen, K. G.; Balle, T.; Bang-Andersen, B. Bioorg. Med. Chem. 2013, 21, 196.[14] Shen, X. Q.; Wu Y. L.; Qian, H. J. Chem. Ind. Times 2012, 26, 29 (in Chinese).(沈学全, 吴金龙, 钱海均, 化工时刊, 2012, 26, 29.)[15] Zhang, X. F.; Liu, H. Y.; Gao, W. T. J. Bohai Univ. (Nat. Sci.) 2009, 30, 212 (in Chinese).(张晓飞, 刘华业, 高文涛, 渤海大学学报(自然科学版), 2009, 30, 212.)[16] Singh, R. P.; Majumder, U.; Shreeve, J. M. J. Org. Chem. 2001, 19, 6263.[17] Lei, J.; Fang, Q.; Yuan, M.-S.; Liu, Z.-Q.; Shen, Y.-X.; Chen, H.-F. Org. Lett. 2010, 22, 5192.[18] Hennessy, E. J.; Buchwald, S. L. J. Am. Chem. Soc. 2003, 40, 12084.[19] Prandi, C.; Occhiato, E. G.; Tabasso, S.; Bonfante, P.; Scarpi, D.; Bova, M. E.; Miletto, I. Eur. J. Org. Chem. 2011, 20, 3781.[20] Hamaue, N.; Mimami, M.; Terado, M.; Hirafuji, M.; Endo, T.; Machida, M.; Hiroshige, T.; Ogata, A.; Tashiro, K.; Saito, H.; Parrez, S. Neurotoxicology 2004. 25, 205.[21] Igosheva, N.; Lorz, C.; Conner, E. Neurochem. Int. 2005, 47, 216.[22] Minami, M.; Hamaue, N.; Hirafuji, M.; Saito, H.; Hiroshige, T.; Ogata, A.; Tashiro, K.; Parvez, S. H. J. Neural Transm. Suppl. 2006, 71, 87.[23] Aulabaugh, A.; Kapoor, B.; Huang, X. Y.; Dollings, P.; Hum, W. T.; Banker, A.; Wood, A.; Ellestad, G. Biochemistry 2007, 46, 9462.[24] Ogata, A.; Hamaue, N.; Terado, M.; Mimami, M.; Nagashima, K.; Tashiro, K. J. Neurol. Sci. 2003, 206, 79.[25] Kenner, C.; Rice, B. J.; Boone, A. B.; Rubin, T. J. J. Med. Chem. 1976, 19, 887.[26] Welstead, Jr.; W. J.; Moran, H. W.; Stauffer, H. F.; Turnbull, L. B.; Sancilio, L. F. J. Med. Chem. 1979, 22, 1074.[27] Rivalle, C.; Bisagni, E. J. Heterocycl. Chem. 1997, 34, 441.[28] Gassman, P. G.; Cue Jr. B. W.; Luh, T. Y. J. Org. Chem. 1977, 42, 1344.[29] Lackey, K.; Besterman, J. M.; Fletcher, W.; Leitner, P.; Morton, B.; Sternbach, D. D. J. Med. Chem. 1995, 38, 906[30] Garden, S. J.; Torees, J.; Ferrira, A. A.; Silva, R. B.; Pinto, A. C. Tetrahedron Lett. 1997, 38, 1501.[31] Kawaguchi, H.; Mizuta, Y.; Sugai, F.; Saito, S. EP 19950308606, 1996 [Chem. Abstr. 1996, 125, 181167].[32] Friedman, S. J. US 3659011, 1972 [Chem. Abstr. 1972, 77, 125053].[33] Marti, C.; Carreira, E. M. J. Am. Chem. Soc. 127, 11505.[34] Cui, X. J.; Shi, F.; Zhang, Y. Tetrahedron Lett. 2010, 51, 2048.[35] Matos, I.; Prez-Mayora, E.; Soriano, E.; Zukal, A.; Martín-Aranda R. M.; López-Peinado, A. J.; Fonseca, I.; ?ejka, J. Chem. Eng. J. 2010, 161, 377.[36] Reddy, C. R.; Jithender, E. Tetrahedron Lett. 2009, 50, 5633.[37] Shah, H. C.; Shah, V. H.; Desai, N. D. ChemInform 2010, 40, 540.[38] Jha, M.; Chou, T. Y.; Blunt, B. Tetrahedron 2011, 67, 982. |