[1] (a) Rottmann, M.; McNamara, C.; Yeung, B. K. S.; Lee, M. C. S.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D. M.; Dharia, N. V.; Tan, J.; Cohen, S. B.; Spencer, K. R.; Gonzalez-Paez, G. E.; Lakshminarayana, S. B.; Goh, A.; Suwanarusk, R.; Jegla, T.; Schmitt, E. K.; Beck, H. P.; Brun, R.; Nosten, F.; Renia, L.; Dartois, V.; Keller, T. H.; Fidock, D. A.; Winzeler, E. A.; Diagana, T. T. Science 2010, 329, 1175.
(b) Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104.
(c) Zheng, Y. J.; Tice, C. M.; Singh, S. B. Bioorg. Med. Chem. Lett. 2014, 24, 3673.
(d) Yu, B.; Yu, D. Q.; Liu, H. M. Eur. J. Med. Chem. 2015, 97, 673.
(e) Ye, N.; Chen, H.; Wold, E. A.; Shi, P.-Y.; Zhou, J. ACS Infect. Dis. 2016, 2, 382.
(f) Mei, G. J.; Shi, F. Chem. Commun. 2018, 54, 6607.
[2] (a) Peddibhotla, S. Curr. Bioact. Compd. 2009, 5, 20.
(b) Cui, B.-D.; Zuo, J.; Zhao, J.-Q.; Zhou, M.-Q.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. J. Org. Chem. 2014, 79, 5305.
[3] (a) Vintonyak, V. V.; Warburg, K.; Kruse, H.; Grimme, S.; Hübel, K.; Rauh, D.; Waldmann, H. Angew. Chem., Int. Ed. 2010, 49, 5902.
(b) Jiang, X.; Cao, Y.; Wang, Y.; Liu, L.; Shen, F.; Wang, R. J. Am. Chem. Soc. 2010, 132, 15328.
(c) Crosignani, S.; Jorand-Lebrun, C.; Page, P.; Campbell, G.; Colovray, V.; Missotten, M.; Humbert, Y.; Cleva, C.; Arrighi, J.-F.; Gaudet, M.; Johnson, Z.; Ferro, P.; Chollet, A. ACS Med. Chem. Lett. 2011, 2, 644.
(d) Rana, S.; Natarajan, A. Org. Biomol. Chem. 2013, 11, 244.
[4] Franz, A. K.; Dreyfuss, P. D.; Schreiber, S. L. J. Am. Chem. Soc. 2007, 129, 1020.
[5] Ghosh, A.; Carter, R. G. Angew. Chem., Int. Ed. 2019, 58, 681.
[6] (a) Alcaide, B.; Almendros, P.; Rodriguez-Acebes, R. J. Org. Chem. 2006, 71, 2346.
(b) Li, J.; Liu, Y. J.; Li, C. J.; Jia, X. Chem.-Eur. J. 2011, 17, 7409.
(c) Hanhan, N. V.; Jones, N. R.; Tran, N. T.; Franz, A. K. Angew. Chem., Int. Ed. 2012, 51, 989.
(d) Yang, L.; Xie, P.; Li, E.; Li, X.; Huang, Y.; Chen, R. Org. Biomol. Chem. 2012, 10, 7628.
(e) Lian, Z.; Shi, M. Eur. J. Org. Chem. 2012, 2012, 581.
(f) Mei, L.-Y.; Wei, Y.; Xu, Q.; Shi, M. Organometallics 2013, 32, 3544.
(g) Tang, Z.; Liu, Z.; An, Y.; Jiang, R.; Zhang, X.; Li, C.; Jia, X.; Li, J. J. Org. Chem. 2016, 81, 9158.
(h) Silvi, M.; Chatterjee, I.; Liu, Y.-K.; Melchiorre, P. Angew. Chem., Int. Ed. 2013, 52, 10780.
(i) Zhao, B.-L.; Du, D.-M. Adv. Synth. Catal. 2016, 358, 3992. (j) Zhu, Y.-S.; Wang, W.-B.; Yuan, B.-B.; Li, Y.-N.; Wang, Q.-L.; Bu, Z.-W. Org. Biomol. Chem. 2017, 15, 984.
(k) Zhang, J.-H.; Wang, R.-B.; Li, D.-F.; Zhao, L.-M. ACS Omega 2017, 2, 7022.
[7] (a) Lee, S.; Hartwig, J. F. J. Org. Chem. 2001, 66, 3402.
(b) Jones, K.; Wilkinson, J. Chem. Commun. 1992, 1767.
(c) Overman, L. E.; Rosen, M. D. Angew. Chem., Int. Ed. 2000, 39, 4596.
(d) Kyei, A. S.; Tchabanenko, K.; Baldwin, J. E.; Adlington, R. M. Tetrahedron Lett. 2004, 45, 8931.
(e) Jaegli, S.; Dufour, J.; Wei, H.-L.; Piou, T.; Duan, X.-H.; Vors, J.-P.; Neuville, L.; Zhu, J.-P. Org. Lett. 2010, 12, 4498.
(f) Yin, B.-L.; Lai, J.-Q.; Zhang, Z.-R.; Jiang, H.-F. Adv. Synth. Catal. 2011, 353, 1961.
[8] (a) Hua, Y.-Z.; Lu, L.-J.; Huang, P.-J.; Wei, D.-H.; Tang, M.-S.; Wang, M.-C.; Chang, J.-B. Chem.-Eur. J. 2014, 20, 12394.
(b) Hua, Y.-Z.; Han, X.-W.; Yang, X.-C.; Song, X.; Wang, M.-C.; Chang, J.-B. J. Org. Chem. 2014, 79, 11690.
(c) Hua, Y.-Z.; Yang, X.-C.; Liu, M.-M.; Song, X.; Wang, M.-C.; Chang, J.-B. Macromolecules 2015, 48, 1651.
(e) Wang, X.-W.; Hua, Y.-Z.; Wang, M.-C. J. Org. Chem. 2016, 81, 9227.
(f) Shao, N.; Luo, Y.-Y.; Lu, H.-J.; Hua, Y.-Z.; Wang, M.-C. Tetrahedron 2018, 74, 2130.
(g) Hua, Y.-Z.; Han, X.-W.; Huang, L.-H.; Wang, M.-C. Chin. J. Org. Chem. 2018, 38, 237(in Chinese). (华远照, 韩兴旺, 黄利华, 王敏灿, 有机化学, 2018, 38, 237.)
(h) Hua, Y.-Z.; Chen, J.-W.; Yang, H.; Wang, M.-C. J. Org. Chem. 2018, 83, 1160.
(i) Zhang, Z.-F.; Yang, X.-C.; Lu, H.-J.; Wang, M.-C. Eur. J. Org. Chem. 2018, 785. (j) Gao, Y.-Y.; Hua, Y.-Z.; Wang, M.-C. Adv. Synth. Catal. 2018, 360, 80.
(k) Liu, S.; Gao, W.-C.; Miao, Y.-H.; Wang, M.-C. J. Org. Chem. 2019, 84, 2652.
[9] (a) Song, X.; Liu, J.; Liu, M.-M.; Wang, X.; Zhang, Z.-F.; Wang, M.-C.; Chang, J. Tetrahedron 2014, 70, 5468.
(b) Hua, Y.-Z.; Liu, M.-M.; Huang, P.-J.; Song, X.; Wang, M.-C.; Chang, J.-B. Chem.-Eur. J. 2015, 21, 11994.
(c) Tao, B.-K.; Yang, H.; Hua, Y.-Z.; Wang, M.-C. Org. Biomol. Chem. 2019, 17, 4301.
(d) Miao, Y.-H.; Hua, Y.-Z.; Wang, M.-C. Org. Biomol. Chem. 2019, 17, 7172.
(e) Liu, M.-M.; Yang, X.-C.; Hua, Y.-Z.; Chang, J.-B.; Wang, M.-C. Org. Lett. 2019, 21, 7089.
(f) Yi, Y.; Hua, Y.-Z.; Lu, H.-J.; Liu, L.-T.; Wang, M.-C. Org. Lett. 2020, 22, 2527.
(g) Liu, M.-M.; Yang, X.-C.; Hua, Y.-Z.; Chang, J.-B.; Wang, M.-C. Org. Lett. 2019, 21, 2111.
(h) Yang, X.-C.; Liu, M.-M.; Mathey, F.; Yang, H.; Hua, Y.-Z.; Wang, M.-C. J. Org. Chem. 2019, 84, 7762.
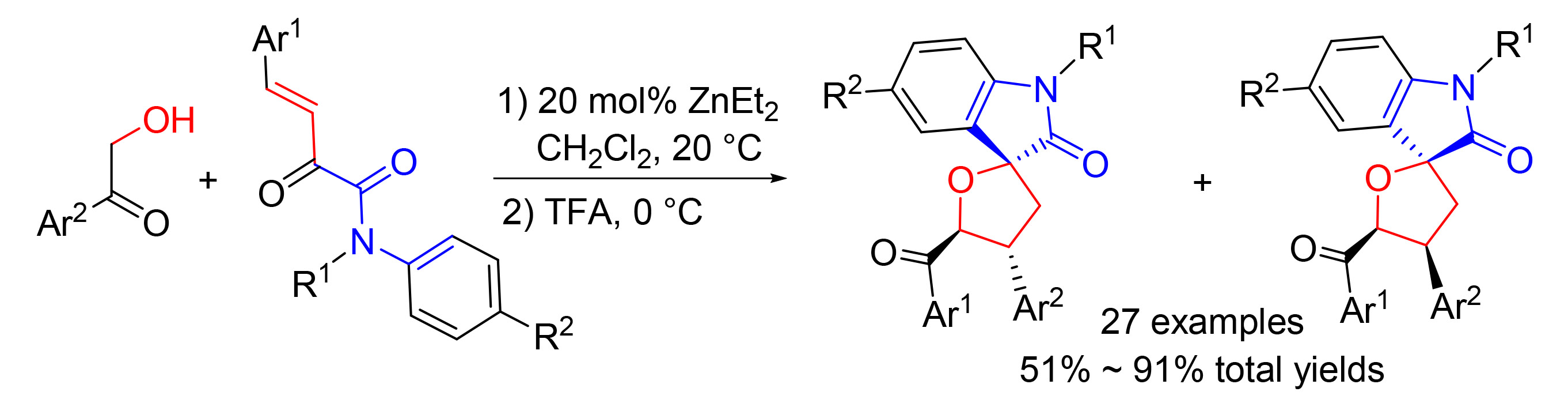
[10] Guo, Y.-J.; Guo, X.; Kong, D.-Z.; Lu, H.-J.; Liu, L.-T.; Hua, Y.-Z.; Wang, M.-C. J. Org. Chem., 2020, 85, 4195. The minor diastereomers in this article are obtained in 23%~44% isolated yields with about 1:1dr value in the current study.
[11] CCDC 1987133(5') contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre. |