有机化学 ›› 2022, Vol. 42 ›› Issue (8): 2527-2534.DOI: 10.6023/cjoc202203055 上一篇 下一篇
研究论文
王苛莉a, 黄静a, 刘伟b, 伍智林a, 于贤勇b, 蒋俊a,*( ), 何卫民a,*(
), 何卫民a,*( )
)
收稿日期:2022-03-28
修回日期:2022-04-26
发布日期:2022-05-06
通讯作者:
蒋俊, 何卫民
基金资助:
Keli Wanga, Jing Huanga, Wei Liub, Zhilin Wua, Xianyong Yub, Jun Jianga( ), Weimin Hea(
), Weimin Hea( )
)
Received:2022-03-28
Revised:2022-04-26
Published:2022-05-06
Contact:
Jun Jiang, Weimin He
Supported by:文章分享
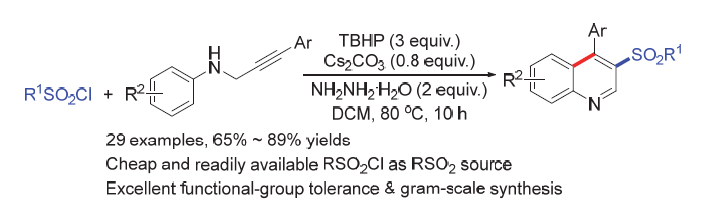
3-砜基喹啉广泛存在于多种生物活性分子和合成药物中. 报道了一种温和条件下, 以N-炔丙基苯胺和磺酰氯为原料, 通过一锅法连续肼化/砜化/环化反应高效构建3-砜基喹啉类化合物的方法. 该方法不仅可以进行克级合成, 还可用于药物分子砜基衍生物的合成.
王苛莉, 黄静, 刘伟, 伍智林, 于贤勇, 蒋俊, 何卫民. 由N-(2-丙炔基)苯胺和磺酰氯直接合成3-砜基喹啉[J]. 有机化学, 2022, 42(8): 2527-2534.
Keli Wang, Jing Huang, Wei Liu, Zhilin Wu, Xianyong Yu, Jun Jiang, Weimin He. Direct Synthesis of 3-Sulfonylquinolines from N-Propargylanilines with Sulfonyl Chlorides[J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2527-2534.
| Entry | Oxidant | Base | Solvent | Temp./℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | TBHP | K2CO3 | DCM | 80 | 58 |
| 2 | DTBP | K2CO3 | DCM | 80 | 11 |
| 3 | BPO | K2CO3 | DCM | 80 | 6 |
| 4 | H2O2 | K2CO3 | DCM | 80 | Trace |
| 5 | PhI(OAc)2 | K2CO3 | DCM | 80 | 8 |
| 6 | K2S2O8 | K2CO3 | DCM | 80 | 14 |
| 7 | TBHP | Na2CO3 | DCM | 80 | 44 |
| 8 | TBHP | Cs2CO3 | DCM | 80 | 90 |
| 9 | TBHP | Et3N | DCM | 80 | 55 |
| 10 | TBHP | Pyridine | DCM | 80 | 43 |
| 11 | TBHP | DABCO | DCM | 80 | 22 |
| 12 | TBHP | Cs2CO3 | DCE | 80 | 69 |
| 13 | TBHP | Cs2CO3 | MeCN | 80 | 82 |
| 14 | TBHP | Cs2CO3 | EtOH | 80 | 9 |
| 15 | TBHP | Cs2CO3 | EtOAc | 80 | 45 |
| 16 | TBHP | Cs2CO3 | THF | 80 | 35 |
| 17 | TBHP | Cs2CO3 | DCM | 90 | 89 |
| 18 | TBHP | Cs2CO3 | DCM | 70 | 62 |
| 19 | — | Cs2CO3 | DCM | 80 | — |
| 20 | TBHP | — | DCM | 80 | 15 |
| 21c | TBHP | Cs2CO3 | DCM | 80 | — |
| 22d | TBHP | Cs2CO3 | DCM | 80 | 84 |
| 22d | TBHP | Cs2CO3 | DCM | 80 | 55 |
| Entry | Oxidant | Base | Solvent | Temp./℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | TBHP | K2CO3 | DCM | 80 | 58 |
| 2 | DTBP | K2CO3 | DCM | 80 | 11 |
| 3 | BPO | K2CO3 | DCM | 80 | 6 |
| 4 | H2O2 | K2CO3 | DCM | 80 | Trace |
| 5 | PhI(OAc)2 | K2CO3 | DCM | 80 | 8 |
| 6 | K2S2O8 | K2CO3 | DCM | 80 | 14 |
| 7 | TBHP | Na2CO3 | DCM | 80 | 44 |
| 8 | TBHP | Cs2CO3 | DCM | 80 | 90 |
| 9 | TBHP | Et3N | DCM | 80 | 55 |
| 10 | TBHP | Pyridine | DCM | 80 | 43 |
| 11 | TBHP | DABCO | DCM | 80 | 22 |
| 12 | TBHP | Cs2CO3 | DCE | 80 | 69 |
| 13 | TBHP | Cs2CO3 | MeCN | 80 | 82 |
| 14 | TBHP | Cs2CO3 | EtOH | 80 | 9 |
| 15 | TBHP | Cs2CO3 | EtOAc | 80 | 45 |
| 16 | TBHP | Cs2CO3 | THF | 80 | 35 |
| 17 | TBHP | Cs2CO3 | DCM | 90 | 89 |
| 18 | TBHP | Cs2CO3 | DCM | 70 | 62 |
| 19 | — | Cs2CO3 | DCM | 80 | — |
| 20 | TBHP | — | DCM | 80 | 15 |
| 21c | TBHP | Cs2CO3 | DCM | 80 | — |
| 22d | TBHP | Cs2CO3 | DCM | 80 | 84 |
| 22d | TBHP | Cs2CO3 | DCM | 80 | 55 |
| [1] |
(a) Cao, M.; Fang, Y.-L.; Wang, Y.-C.; Xu, X.-J.; Xi, Z.-W.; Tang, S. ACS Comb. Sci. 2020, 22, 268.
doi: 10.1021/acscombsci.0c00012 |
|
(b) Wu, Y.; Chen, J.-Y.; Ning, J.; Jiang, X.; Deng, J.; Deng, Y.; Xu, R.; He, W.-M. Green Chem. 2021, 23, 3950.
doi: 10.1039/D1GC00562F |
|
|
(c) Yang, W.-C.; Chen, C.-Y.; Li, J.-F.; Wang, Z.-L. Chin. J. Catal. 2021, 42, 1865.
doi: 10.1016/S1872-2067(21)63814-7 |
|
|
(d) Chen, S.-F.; Liu, X.-C.; Xu, J.-K.; Li, L.; Lang, J.-J.; Wen, G.-B.; Lin, Y.-W. Inorg. Chem. 2021, 60, 2839.
doi: 10.1021/acs.inorgchem.0c03777 |
|
|
(e) Tong, S.; Li, K.; Ouyang, X.; Song, R.; Li, J. Green Synth. Catal. 2021, 2, 145.
|
|
| [2] |
(a) Ivachtchenko, A. V.; Golovina, E. S.; Kadieva, M. G.; Mitkin, O. D.; Okun, I. M. Pharm. Chem. J. 2015, 48, 646.
doi: 10.1007/s11094-015-1164-5 pmid: 26774652 |
|
(b) Galambos, J.; Domány, G.; Nógrádi, K.; Wágner, G.; Keserű, G. M.; Bobok, A.; Kolok, S.; Mikó-Bakk, M. L.; Vastag, M.; Sághy, K.; Kóti, J.; Szakács, Z.; Béni, Z.; Gál, K.; Szombathelyi, Z.; Greiner, I. Bioorg. Med. Chem. Lett. 2016, 26, 1249.
doi: 10.1016/j.bmcl.2016.01.024 pmid: 26774652 |
|
| [3] |
(a) Deng, Q.; Xu, Y.; Liu, P.; Tan, L.; Sun, P. Org. Chem. Front. 2018, 5, 19.
doi: 10.1039/C7QO00714K |
|
(b) Li, X.-F.; Zhang, X.-G.; Hu, B.-L.; Zhang, X.-H. Org. Biomol. Chem. 2018, 16, 1736.
doi: 10.1039/C8OB00133B |
|
|
(c) Chen, W.; Zhang, Y.; Li, P.; Wang, L. Org. Chem. Front. 2018, 5, 855.
doi: 10.1039/C7QO01052D |
|
|
(d) Yang, M.; Hu, X.; Ouyang, B.; Xie, W.; Liu, J.-B. Tetrahedron 2019, 75, 3516.
doi: 10.1016/j.tet.2019.05.016 |
|
|
(e) Zhou, N.; Xia, Z.; Wu, S.; Kuang, K.; Xu, Q.; Zhang, M. J. Org. Chem. 2021, 86, 15253.
doi: 10.1021/acs.joc.1c01866 |
|
| [4] |
(a) Feng, Y.; Zhang, Z.; Fu, Q.; Yao, Q.; Huang, H.; Shen, J.; Cui, X. Chin. Chem. Lett. 2020, 31, 58.
doi: 10.1016/j.cclet.2019.05.013 |
|
(b) Tang, L.; Du, K.; Yu, B.; He, L. Chin. Chem. Lett. 2020, 31, 2991.
doi: 10.1016/j.cclet.2020.03.030 |
|
|
(c) Lv, Y.; Cui, H.; Meng, N.; Yue, H.; Wei, W. Chin. Chem. Lett. 2022, 33, 97.
doi: 10.1016/j.cclet.2021.06.068 |
|
|
(d) Wang, Z.; Liu, Q.; Liu, R.; Ji, Z.; Li, Y.; Zhao, X.; Wei, W. Chin. Chem. Lett. 2022, 33, 1479.
doi: 10.1016/j.cclet.2021.08.036 |
|
| [5] |
(a) Tang, S.; Deng, Y.-L.; Li, J.; Wang, W.-X.; Wang, Y.-C.; Li, Z.-Z.; Yuan, L.; Chen, S.-L.; Sheng, R.-L. Chem. Commun. 2016, 52, 4470.
doi: 10.1039/C5CC10464E pmid: 29565432 |
|
(b) Yuan, L.; Jiang, S.-M.; Li, Z.-Z.; Zhu, Y.; Yu, J.; Li, L.; Li, M.-Z.; Tang, S.; Sheng, R.-R. Org. Biomol. Chem. 2018, 16, 2406.
doi: 10.1039/C8OB00132D pmid: 29565432 |
|
|
(c) Yu, J.; Sheng, H.-X.; Wang, S.-W.; Xu, Z.-H.; Tang, S.; Chen, S.-L. Chem. Commun. 2019, 55, 4578.
doi: 10.1039/C9CC00294D pmid: 29565432 |
|
|
(d) Gui, Q.-W.; Teng, F.; Yang, H.; Xun, C.; Huang, W.-J.; Lu, Z.-Q.; Zhu, M.-X.; Ouyang, W.-T.; He, W.-M. Chem. Asian J. 2022, 17, e202101139.
pmid: 29565432 |
|
| [6] |
(a) Li, L.; Zhang, X.-G.; Hu, B.-L.; Zhang, X.-H. Chem. Asian J. 2019, 14, 4358.
doi: 10.1002/asia.201901298 |
|
(b) Yuan, J.-M.; Li, J.; Zhou, H.; Xu, J.; Zhu, F.; Liang, Q.; Liu, Z.; Huang, G.; Huang, J. New J. Chem. 2020, 44, 3189.
doi: 10.1039/C9NJ05248H |
|
| [7] |
(a) Zhang, Y.; Chen, W.; Jia, X.; Wang, L.; Li, P. Chem. Commun. 2019, 55, 2785.
doi: 10.1039/C8CC10235J |
|
(b) Liu, J.; Wang, M.; Li, L.; Wang, L. Green Chem. 2021, 23, 4733.
doi: 10.1039/D1GC00171J |
|
| [8] |
Zhang, L.; Chen, S.; Gao, Y.; Zhang, P.; Wu, Y.; Tang, G.; Zhao, Y. Org. Lett. 2016, 18, 1286.
doi: 10.1021/acs.orglett.6b00198 |
| [9] |
Sun, D.; Yin, K.; Zhang, R. Chem. Commun. 2018, 54, 1335.
doi: 10.1039/C7CC09410H |
| [10] |
Liu, Q.; Mei, Y.; Wang, L.; Ma, Y.; Li, P. Adv. Synth. Catal. 2020, 362, 5669.
doi: 10.1002/adsc.202000846 |
| [11] |
(a) Zhu, C.; Han, M.-Y.; Liang, X.-X.; Guan, B.; Li, P.; Wang, L. Org. Lett. 2021, 23, 54.
doi: 10.1021/acs.orglett.0c03683 pmid: 25858623 |
|
(b) Zhou, D.; Li, Z.-H.; Li, J.; Li, S.-H.; Wang, M.-W.; Luo, X.-L.; Ding, G.-L.; Sheng, R.-L.; Fu, M.-J.; Tang, S. Eur. J. Org. Chem. 2015, 2015, 1606.
doi: 10.1002/ejoc.201403499 pmid: 25858623 |
|
|
(c) Tang, S.; Li, Z.-H.; Wang, M.-W.; Li, Z.-P.; Sheng, R.-L. Org. Biomol. Chem. 2015, 13, 5285.
doi: 10.1039/c5ob00454c pmid: 25858623 |
|
|
(d) Ma, C.-H.; Ji, Y.; Zhao, J.; He, X.; Zhang, S.-T.; Jiang, Y.-Q.; Yu, B. Chin. J. Catal. 2022, 43, 571.
doi: 10.1016/S1872-2067(21)63917-7 pmid: 25858623 |
|
| [12] |
(a) Chen, J.-Y.; Wu, H.-Y.; Gui, Q.-W.; Yan, S.-S.; Deng, J.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. J. Catal. 2021, 42, 1445.
doi: 10.1016/S1872-2067(20)63750-0 |
|
(b) Yin, L. L.; Yuan, H.; Liu, C.; He, B.; Gao, S.-Q.; Wen, G.-B.; Tan, X.; Lin, Y.-W. ACS Catal. 2018, 8, 9619.
doi: 10.1021/acscatal.8b02979 |
|
|
(c) Chen, X.; Xiao, F.; He, W.-M. Org. Chem. Front. 2021, 8, 5206.
doi: 10.1039/D1QO00375E |
|
|
(d) Wu, Y.; Chen, J.-Y.; Liao, H.-R.; Shu, X.-R.; Duan, L.-L.; Yang, X.-F.; He, W.-M. Green Synth. Catal. 2021, 2, 233.
|
|
|
(e) Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Chin. J. Catal. 2021, 42, 1921.
doi: 10.1016/S1872-2067(21)63850-0 |
|
|
(f) Wu, Z.-L.; Chen, J.-Y.; Tian, X.-Z.; Ouyang, W.-T.; Zhang, Z.-T.; He, W.-M. Chin. Chem. Lett. 2022, 33, 1501.
doi: 10.1016/j.cclet.2021.08.071 |
| [1] | 江港钟, 林嘉欣, 鲍晓光, 万小兵. 亚硝酸异戊酯活化伯磺酰胺制备磺酰溴与磺酰氯[J]. 有机化学, 2024, 44(2): 533-549. |
| [2] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [3] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [4] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [5] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [6] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [7] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [8] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [9] | 王永玲, 张铁欣, 张栩铭, 孙晗扬, 冷津瑶, 李亚明. 可见光催化N-芳基乙醛酸亚胺脱羧烷基化合成非天然氨基酸衍生物[J]. 有机化学, 2023, 43(12): 4284-4293. |
| [10] | 景智霞, 杜建喜, 蒋平, 阿布拉江•克依木. 四丁基碘化胺介导烷基酰胺与酰肼一锅法构建1,3,4-噁二唑衍生物[J]. 有机化学, 2023, 43(11): 3930-3938. |
| [11] | 南宁, 吴双, 秦景灏, 李金恒. 基于硅烷化启动的环化反应研究进展[J]. 有机化学, 2023, 43(10): 3414-3453. |
| [12] | 乃比江•赛米, 张蕾, 买地娜•沙拉木, 曾竟, 阿布都热西提•阿布力克木. 硫代磺酸酯和磺酰卤的绿色合成研究[J]. 有机化学, 2023, 43(1): 236-243. |
| [13] | 覃小婷, 邹宁, 农彩梅, 莫冬亮. 九元氮杂环化合物合成最新研究进展[J]. 有机化学, 2023, 43(1): 130-155. |
| [14] | 桑田, 贾帆, 何静, 李春天, 刘岩, 刘平. I2催化β-酮腈与1H-吡唑-5-胺的环化反应[J]. 有机化学, 2023, 43(1): 195-201. |
| [15] | 刘东汉, 鲁席杭, 柴张梦洁, 杨浩琦, 孙瑜琳, 余富朝. 构建2H-吡咯-2-酮骨架的研究进展[J]. 有机化学, 2023, 43(1): 57-73. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||