有机化学 ›› 2021, Vol. 41 ›› Issue (9): 3448-3458.DOI: 10.6023/cjoc202104060 上一篇 下一篇
综述与进展
收稿日期:2021-04-30
修回日期:2021-05-25
发布日期:2021-06-07
通讯作者:
王鹏
基金资助:
Peng Wanga( ), Da Yangb, Huan Liub
), Da Yangb, Huan Liub
Received:2021-04-30
Revised:2021-05-25
Published:2021-06-07
Contact:
Peng Wang
Supported by:文章分享

β-内酰胺类化合物是具有高生物活性及高应用价值的抗生素, 如何高效高选择性地设计、合成该类化合物一直是有机化学研究的热点问题. 由于其环状结构中具有羰基结构, 利用一氧化碳(CO)作为羰源与底物分子发生羰基化反应也发展成为合成β-内酰胺的有效方法. 通过该方法可以一步高效合成结构多样性且新颖的β-内酰胺化合物. 综述了近年来通过不同底物分子与CO发生羰基化反应构建β-内酰胺的研究进展, 并且对该方法存在的问题以及未来发展方向进行了展望.
王鹏, 杨妲, 刘欢. 一氧化碳参与β-内酰胺化合物合成的研究进展[J]. 有机化学, 2021, 41(9): 3448-3458.
Peng Wang, Da Yang, Huan Liu. Recent Advances on the Synthesis of β-Lactams by Involving Carbon Monoxide[J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3448-3458.
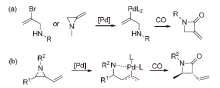
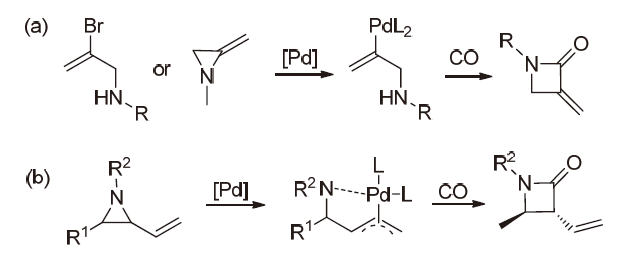

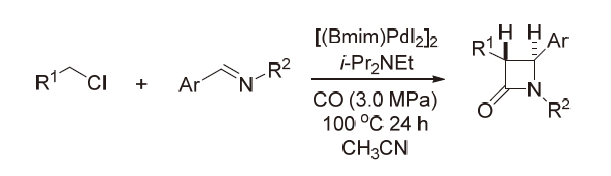
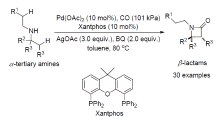
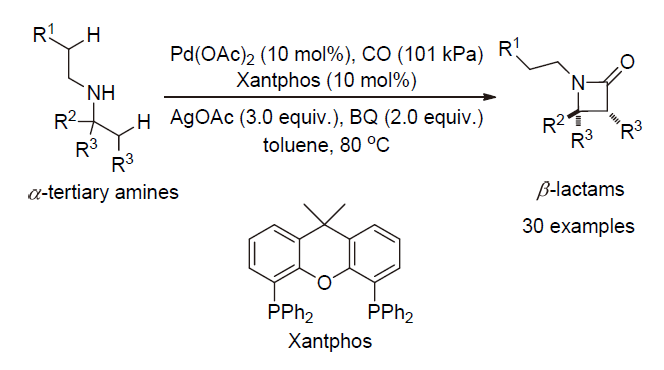


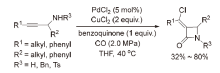
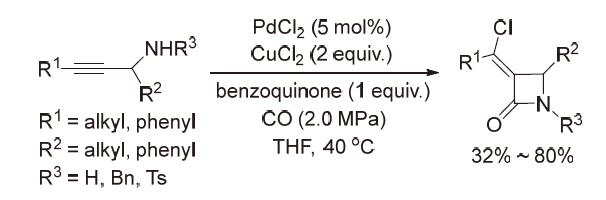
| [1] |
Fleming, A. Br. J. Exp. Pathol. 1929, 10, 226.
|
| [2] |
Brandi, A.; Cicchi, S.; Cordero, F. M. Chem. Rev. 2008, 108, 3988.
doi: 10.1021/cr800325e |
| [3] |
Cossío, F. P.; Arrieta, A.; Sierra, M. A. Acc. Chem. Res. 2008, 41, 925.
doi: 10.1021/ar800033j |
| [4] |
Liang, Y.; Jiao, L.; Zhang, S.; Yu, Z. X.; Xu, J. J. Am. Chem. Soc. 2009, 131, 1542.
doi: 10.1021/ja808046e pmid: 19132931 |
| [5] |
Liu, M. S.; Fu, N. Y. Chin. J. Org. Chem. 2010, 30, 499. (in Chinese).
|
|
( 刘明舜, 傅南雁, 有机化学, 2010, 30, 499.)
|
|
| [6] |
Maree, C. L.; Daum, R. S.; Boyle-Vavra, S.; Matayoshi, K.; Miller, L. G. Emerging Infect. Dis. 2007, 13, 236.
doi: 10.3201/eid1302.060781 |
| [7] |
Ojima, I.; Delaloge, F. Chem. Soc. Rev. 1997, 26, 377.
doi: 10.1039/CS9972600377 |
| [8] |
Tuba, R. Org. Biomol. Chem. 2013, 11, 5976.
doi: 10.1039/c3ob41048j |
| [9] |
Doyle, M. P.; Forbes, D. C. Chem. Rev. 1998, 98, 911.
doi: 10.1021/cr940066a |
| [10] |
Cheung, W. H.; Zheng, S. L.; Yu, W. Y.; Zhou, G. C.; Che, C. M. Org. Lett. 2003, 5, 2535.
doi: 10.1021/ol034806q |
| [11] |
Choi, M. K. W.; Yu, W. Y.; Che, C. M. Org. Lett. 2005, 7, 1081.
doi: 10.1021/ol050003m |
| [12] |
Brandi, A.; Cicchi, S.; Cordero, F. M. Chem. Rev. 2008, 108, 3988.
doi: 10.1021/cr800325e |
| [13] |
Lo, M. M. C.; Fu, G. C. J. Am. Chem. Soc. 2002, 124, 4572.
doi: 10.1021/ja025833z |
| [14] |
Shintani, R.; Fu, G. C. Angew. Chem., Int. Ed. 2003, 42, 4082.
doi: 10.1002/(ISSN)1521-3773 |
| [15] |
Saito, T.; Kikuchi, T.; Tanabe, H.; Yahiro, J.; Otani, T. Tetrahedron Lett. 2009, 50, 4969.
doi: 10.1016/j.tetlet.2009.06.050 |
| [16] |
Gilman, H.; Speeter, M. J. Am. Chem. Soc. 1943, 65, 2255.
|
| [17] |
Benaglia, M.; Cinquini, M.; Cozzi, F. Eur. J. Org. Chem. 2000, 563.
|
| [18] |
Beller, M.; Wu, X. F. Transition Metal Catalyzed Carbonylation Reactions: Carbonylative Activation of C-X Bonds, Springer, Amsterdam, 2013.
|
| [19] |
Nienburg, H. J.; Elschnigg, G. Chem. Abstr. 1961, 55, 10323h.
|
| [20] |
Piens, N.; D'hooghe, M. Eur. J. Org. Chem. 2017, 40, 5943.
|
| [21] |
Mele, G. G. Curr. Org. Chem. 2006, 10, 1397.
doi: 10.2174/138527206778018276 |
| [22] |
Huang, C. Y. D.; Doyle, A. G. Chem. Rev. 2014, 114, 8153.
doi: 10.1021/cr500036t |
| [23] |
Alper, H.; Urso, F. J. Am. Chem. Soc. 1983, 105, 6737.
doi: 10.1021/ja00360a045 |
| [24] |
Alper, H.; Hamel, N. Tetrahedron Lett. 1987, 28, 3237.
doi: 10.1016/S0040-4039(00)95481-9 |
| [25] |
Calet, S.; Urso, F.; Alper, H. J. Am. Chem. Soc. 1989, 11, 931.
|
| [26] |
Spears, G. W.; Nakanishi, K.; Ohfune, Y. Synlett 1991, 91.
|
| [27] |
Tanner, D.; Somfaib, P. Bioorg. Med. Chem. Lett. 1993, 3, 2415.
doi: 10.1016/S0960-894X(01)80967-7 |
| [28] |
Tanner, D.; Somfaib, P. Tetrehedron 1988, 44, 619.
doi: 10.1016/S0040-4020(01)85849-X |
| [29] |
Piotti, M. E.; Alper, H. J. Am. Chem. Soc. 1989, 11, 931.
|
| [30] |
Davoli, P.; Forni, A.; Moretti, I.; Prati, F.; Torre, G. Tetrahedron 2001, 57, 1801.
doi: 10.1016/S0040-4020(00)01152-2 |
| [31] |
Chamchaang, W.; Pinhas, A. R. J. Chem. Soc., Chem. Commun. 1998, 61, 710.
|
| [32] |
Mahadevan, V.; Getzler, Y. D. Y. L.; Coates, G. W. Angew. Chem., Int. Ed. 2002, 41, 2781.
doi: 10.1002/1521-3773(20020802)41:15【-逻*辑*与-】#x00026;lt;2781::AID-ANIE2781【-逻*辑*与-】#x00026;gt;3.0.CO;2-S |
| [33] |
Ardura, D.; Lopez, R.; Sordo, T. L. J. Org. Chem. 2006, 71, 7315.
pmid: 16958525 |
| [34] |
Fontana, F.; Tron, G. C.; Barbero, N.; Ferrini, S.; Thomas, S. P.; Aggarwal, V. K. Chem. Commun. 2010, 46, 267.
doi: 10.1039/B920564K |
| [35] |
Piens, N.; Hecke, K. V.; Vogt, D.; D'hooghe, M. Org. Biomol. Chem. 2017, 15, 4816.
doi: 10.1039/C7OB00832E |
| [36] |
Staudinger, H. Justus Liebigs Ann. Chem. 1907, 356, 51.
doi: 10.1002/(ISSN)1099-0690 |
| [37] |
Tidwell, T. T. Ketenes, 2nd ed., John Wiley and Sons, Hoboken, NJ, 2006.
|
| [38] |
Torii, S.; Okumoto, H.; Sadakane, M.; Hai, A. K. M. A.; Tanaka, H. Tetrahedron Lett. 1993, 34, 6553.
doi: 10.1016/0040-4039(93)88102-O |
| [39] |
Tanaka, H.; Hai, A. K. M. A.; Sadakane, M.; Okumoto, H.; Torii, S. J. Org. Chem. 1994, 59, 3040.
doi: 10.1021/jo00090a023 |
| [40] |
Troisi, L.; Vitis, L. D.; Granito, C.; Pilati, T.; Pindinelli, E. Tetrahedron 2004, 60, 6895.
doi: 10.1016/j.tet.2004.05.079 |
| [41] |
Troisi, L.; Vitis, L. D.; Granito, C.; Epifani, E. Eur. J. Org. Chem. 2004, 1357.
|
| [42] |
Wartski, L. Bull. Soc. Chim. Fr. 1975, 1663.
|
| [43] |
Dhawan, R.; Dghaym, R. D.; Cyr, D. J. S.; Arndtsen, B. A. J. Org. Chem. 2006, 8, 3927.
|
| [44] |
Troisi, L.; Pindinelli, E.; Strusi, V.; Trinchera, P. Tetrahedron: Asymmetry 2009, 20, 368.
|
| [45] |
Clarke, J. F.; Gerard, G. W. A.; David, A. J. Org. Chem. 1974, 74, 417.
|
| [46] |
Vaccari, D.; Davoli, P.; Spaggiari, A.; Prati, F. Synlett 2008, 1317.
|
| [47] |
Zhang, Z. H.; Liu, Y. Y.; Ling, L.; Li, Y. X.; Dong, Y. A.; Gong, M. X.; Zhao, X. K.; Zhang, Y.; Wang, J. B. J. Am. Chem. Soc. 2011, 133, 4330.
doi: 10.1021/ja107351d |
| [48] |
Xie, P.; Qian, B.; Huang, H. M.; Xia, C. G. Tetrahedron Lett. 2012, 53, 1613.
doi: 10.1016/j.tetlet.2012.01.073 |
| [49] |
Li, L. L.; Ding, D.; Song, J.; Han, Z. Y.; Gong, L. Z. Angew. Chem., Int. Ed. 2019, 58, 7647.
doi: 10.1002/anie.v58.23 |
| [50] |
Ye, T.; McKervey, M. A. Chem. Rev. 1994, 94, 1091.
doi: 10.1021/cr00028a010 |
| [51] |
Doyle, M. P.; McKervey, M. A.; Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley-Inter-science, New York, 1998.
|
| [52] |
Zhang, Z.; Wang, J. Tetrahedron 2008, 64, 6577.
doi: 10.1016/j.tet.2008.04.074 |
| [53] |
Zhang, Z. H.; Zhang, Y.; Wang J. B. ACS Catal. 2011, 1, 1621.
doi: 10.1021/cs200434s |
| [54] |
Wentrup, C.; Heilmayer, W.; Kollenz, G. Synthesis 1994, 1219.
|
| [55] |
Tidwell, T. T. Angew. Chem., Int. Ed. 2005, 44, 5778.
doi: 10.1002/(ISSN)1521-3773 |
| [56] |
Tidwell, T. T. Eur. J. Org. Chem. 2006, 563.
|
| [57] |
Fördős, E.; Tuba, R.; Párkányi, L.; Kégl, T.; Ungváry, F. Eur. J. Org. Chem. 2009, 74, 1994.
|
| [58] |
Paul, N. D.; Chirila, A.; Lu, H. J.; Zhang, X. P.; Bruin, B. Chem.- Eur. J. 2013, 19, 12953.
doi: 10.1002/chem.v19.39 |
| [59] |
Tang, Z.; Mandal, S.; Paul, N. D.; Lutz, M.; Li, P.; Vlugt, J. I.; Bruin, B. Org. Chem. Front. 2015, 2, 1561.
doi: 10.1039/C5QO00287G |
| [60] |
Pedroni, J.; Boghi, M.; Saget, T.; Cramer, N. Angew. Chem., Int. Ed. 2014, 53, 9064.
doi: 10.1002/anie.201405508 |
| [61] |
McNally, A.; Haffemayer, B.; Collins, B. S. L. Gaunt, M. J. Science 2014, 510, 129.
|
| [62] |
Willcox, D.; Chappell, B. G. N.; Hogg, K. F.; Calleja, J.; Smalley, A. P.; Gaunt, M. J. Science 2016, 354, 851.
pmid: 27856900 |
| [63] |
Dailler, D.; Rocaboy, R.; Baudoin, O. Angew. Chem., Int. Ed. 2017, 56, 7218.
doi: 10.1002/anie.201703109 |
| [64] |
Zhang, Q.; Chen, K.; Rao, W.; Zhang, Y.; Chen, F. J.; Shi, B. F. Angew. Chem., Int. Ed. 2013, 52, 13588.
doi: 10.1002/anie.201306625 |
| [65] |
Sun, W. W.; Cao, P.; Mei, R. Q.; Li, Y.; Ma, Y. L.; Wu, B. Org. Lett. 2014, 16, 480.
doi: 10.1021/ol403364k |
| [66] |
Zhang, S. J.; Sun, W. W.; Cao, P.; Dong, X. P.; Liu, J. K.; Wu, B. J. Org. Chem. 2016, 81, 956.
doi: 10.1021/acs.joc.5b02532 |
| [67] |
Zhang, Q.; Chen, K.; Shi, B. F. Synlett 2014, 25, 1941.
doi: 10.1055/s-00000083 |
| [68] |
Cabrera-Pardo, J. R.; Trowbridge, A.; Nappi, M.; Ozaki, K.; Gaunt, M. J. Angew. Chem., Int. Ed. 2017, 56, 11958.
doi: 10.1002/anie.201706303 |
| [69] |
Hogg, K. F.; Trowbridge, A.; Perezand, A. A.; Gaunt, M. J. Chem. Sci. 2017, 8, 8189.
|
| [70] |
Mori, M.; Chiba, K.; Okita, M.; Ban, Y. J. Chem. Soc., Chem. Commun. 1979, 698.
|
| [71] |
Chiba, K.; Mori, M.; Ban, Y. Tetrahedron 1985, 41, 387.
doi: 10.1016/S0040-4020(01)96430-0 |
| [72] |
Mori, M.; Chiba, K.; Okita, M.; Kayo, I.; Ban, Y. Tetrahedron 1985, 41, 375.
doi: 10.1016/S0040-4020(01)96429-4 |
| [73] |
Matsuda, I.; Sakakibara, J.; Nagashima, H. Tetrahedron Lett. 1991, 32, 7431.
doi: 10.1016/0040-4039(91)80126-Q |
| [74] |
Zhou, Z. X.; Alper, H. J. Org. Chem. 1996, 61, 1256.
doi: 10.1021/jo9517104 |
| [75] |
Ma, S. M.; Wu, B.; Jiang, X. F. J. Org. Chem. 2005, 70, 2588.
doi: 10.1021/jo0480996 |
| [76] |
Aronica, L. A.; Caporusso, A. M.; Evangelisti, C.; Botavina, M.; Alberto, G.; Martra, G. J. Organomet. Chem. 2012, 700, 20.
doi: 10.1016/j.jorganchem.2011.11.008 |
| [77] |
Li, W.; Liu, C.; Zhang, H.; Ye, K. Y.; Zhang, G. H.; Zhang, W. Z.; Duan, Z. L.; You, S. L.; Lei, A. W. Angew. Chem., Int. Ed. 2014, 53, 2443.
doi: 10.1002/anie.201309081 |
| [78] |
Torres, G. M.; Macias, M. H.; Quesnel, J. S.; Williams, O. P.; Yempally, V.; Bengali, A. A.; Arndtsen, B. A. J. Org. Chem. 2016, 81, 12106.
doi: 10.1021/acs.joc.6b02405 |
| [1] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [2] | 席敏, 段超, 迟捷, 付甜, 苏小龙, 王宏社. 腐殖酸作用下Strecker反应快速高效合成α-氨基腈[J]. 有机化学, 2023, 43(9): 3312-3318. |
| [3] | 陈祖良, 魏颖静, 张俊良. 供体-受体氮杂环丙烷碳-碳键断裂的环加成反应研究进展[J]. 有机化学, 2023, 43(9): 3078-3088. |
| [4] | 吴文倩, 陈春霞, 彭进松, 李占宇. 羰基α-位胺化反应研究进展[J]. 有机化学, 2023, 43(8): 2743-2763. |
| [5] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [6] | 安大列, 包志鹏, 吴小锋. 含碳氟类底物参与的羰基化反应研究进展[J]. 有机化学, 2023, 43(7): 2304-2312. |
| [7] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [8] | 张建涛, 邓雅文, 莫诺琳, 陈莲芬. 自由基介导的α,α-二芳基烯丙醇1,2-芳基迁移反应研究进展[J]. 有机化学, 2023, 43(2): 426-435. |
| [9] | 王维, 张哲宇, 张雪, 于海丰, 罗辉, 霍东月, 徐玉澎, 赵晓波. 多取代2,3-二氢-4-吡啶酮的水相合成[J]. 有机化学, 2023, 43(2): 742-750. |
| [10] | 刘鹏, 钟富明, 廖礼豪, 谭伟强, 赵晓丹. 炔烃参与的去芳构化反应构建螺环己二烯酮类化合物的研究进展[J]. 有机化学, 2023, 43(12): 4019-4035. |
| [11] | 郝二军, 丁笑波, 王珂新, 周红昊, 杨启亮, 石磊. 氮杂环丙烷与不饱和化合物发生[3+2]扩环反应的研究进展[J]. 有机化学, 2023, 43(12): 4057-4074. |
| [12] | 郭广青, 练仲. 硅基羧酸在有机合成中的应用进展[J]. 有机化学, 2023, 43(10): 3580-3589. |
| [13] | 郭泽, 吴迪, 王丽丽, 段征. BF3•Et2O促进的双烯酮-酚重排合成具有聚集诱导发光(AIE)效应的磷杂七元环化合物[J]. 有机化学, 2022, 42(8): 2481-2487. |
| [14] | 冉龙玉, 张成潘. 三氟甲磺酸三氟甲酯的反应研究进展[J]. 有机化学, 2022, 42(7): 2045-2054. |
| [15] | 马志伟, 陈晓培, 王川川, 王建玲, 陶京朝, 吕全建. 手性方酰胺催化环状1,3-二羰基化合物对β,γ-不饱和-α-酮酯的不对称Michael加成反应[J]. 有机化学, 2022, 42(5): 1520-1526. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||