有机化学 ›› 2021, Vol. 41 ›› Issue (12): 4610-4622.DOI: 10.6023/cjoc202107042 上一篇 下一篇
综述与进展
李丹丹*( ), 王晓辰, 李闪闪, 付晨雨, 李倩倩, 许东涛, 马莹莹
), 王晓辰, 李闪闪, 付晨雨, 李倩倩, 许东涛, 马莹莹
收稿日期:2021-07-18
修回日期:2021-09-04
发布日期:2021-09-14
通讯作者:
李丹丹
基金资助:
Dandan Li( ), Xiaochen Wang, Shanshan Li, Chenyu Fu, Qianqian Li, Dongtao Xu, Yingying Ma
), Xiaochen Wang, Shanshan Li, Chenyu Fu, Qianqian Li, Dongtao Xu, Yingying Ma
Received:2021-07-18
Revised:2021-09-04
Published:2021-09-14
Contact:
Dandan Li
Supported by:文章分享
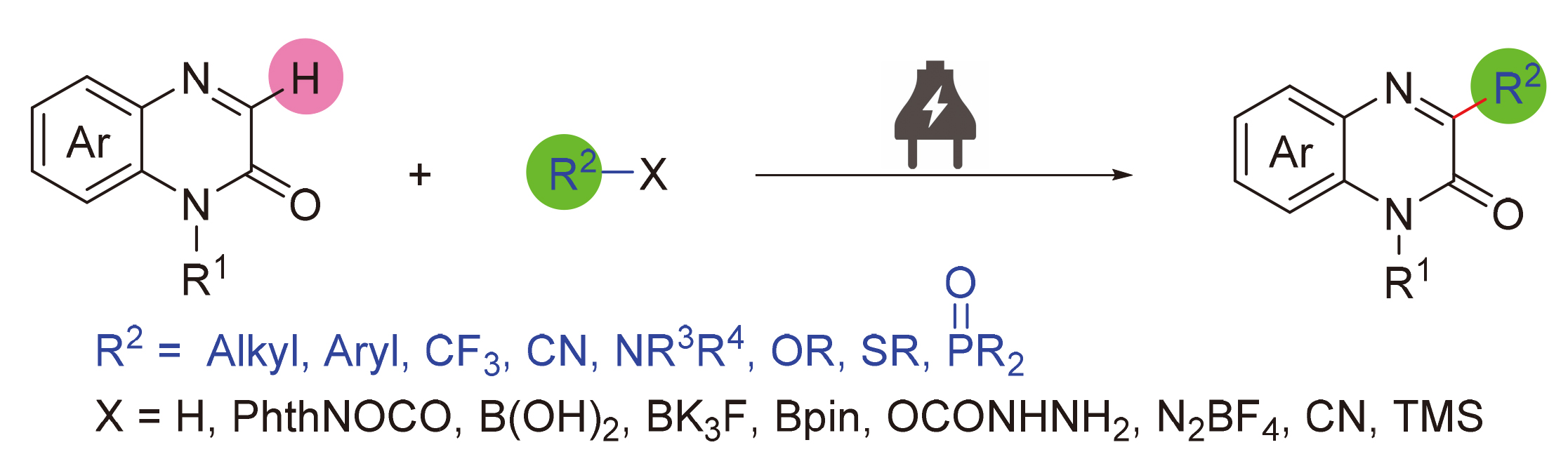
喹喔啉酮及其衍生物是一类重要的氮杂环化合物, 广泛存在于具有各种生物活性的天然产物、药物及功能材料中, 因此, 喹喔啉酮的C—H功能化引起了化学工作者的广泛关注. 电化学合成无需外加氧化还原试剂, 直接利用电子这一“清洁试剂”参与氧化还原反应, 具有反应条件温和及原子经济性好等特点, 十分符合绿色化学符合绿色化学和可持续发展的要求. 随着对电化学合成反应机理的深入研究以及反应设备的标准化, 该方法已经成为喹喔啉酮类化合物功能化的强有力工具. 综述了有机电化学在喹喔啉-2(1H)-酮C(3)—H功能化领域所取得的研究进展, 对反应的条件和机理进行了系统的总结, 并对该领域所面临的挑战及发展方向进行了展望与探讨.
李丹丹, 王晓辰, 李闪闪, 付晨雨, 李倩倩, 许东涛, 马莹莹. 电化学条件下喹喔啉-2(1H)-酮C(3)—H功能化的研究进展[J]. 有机化学, 2021, 41(12): 4610-4622.
Dandan Li, Xiaochen Wang, Shanshan Li, Chenyu Fu, Qianqian Li, Dongtao Xu, Yingying Ma. Recent Advances in Electrochemical C(3)—H Functionalization of Quinoxalin-2(1H)-ones[J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4610-4622.
| [1] |
(a) Waring, M. J.; Ben-Hadda, T.; Kotchevar, A. T.; Ramdani, A.; Touzani, R.; Elkadiri, S.; Hakkou, A.; Bouakka, M.; Ellis, T. Molecules 2002, 7, 641.
doi: 10.3390/70800641 pmid: 24793885 |
|
(b) Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893.
doi: 10.1021/cr020033s pmid: 24793885 |
|
|
(c) Refaat, H. M.; Moneer, A. A.; Khalil, O. M. Arch. Pharm. Res. 2004, 27, 1093.
pmid: 24793885 |
|
|
(d) Carta, A.; Piras, S.; Loriga, G.; Paglietti, G. Mini-Rev. Med. Chem. 2006, 6, 1179.
pmid: 24793885 |
|
|
(e) El-Hawash, S. A. M.; Habib, N. S.; Kassem, M. A. Arch. Pharm. 2006, 339, 564.
pmid: 24793885 |
|
|
(f) Meyer, E.; Joussef, A. C.; de Souza, L. D. B. P. Synth. Commun. 2006, 36, 729.
doi: 10.1080/00397910500447066 pmid: 24793885 |
|
|
(g) Moarbess, G.; Deleuze-Masquefa, C.; Bonnard, V.; Gayraud- Paniagua, S.; Vidal, J.-R.; Bressolle, F.; Pinguet, F.; Bonnet, P.-A. Bioorg. Med. Chem. 2008, 16, 6601.
doi: 10.1016/j.bmc.2008.05.022 pmid: 24793885 |
|
|
(h) Ramli, Y.; Benzeid, H.; Bouhfid, R.; Rodi, Y. K.; Ferfra, S.; Essassi, E. M. Stud. Cercet. Stiint.: Chim. Ing. Chim., Biotehnol., nd. Aliment. 2010, 11, 67.
pmid: 24793885 |
|
|
(i) Liu, R.; Huang, Z.; Murray, M. G.; Guo, X.; Liu, G. J. Med. Chem. 2011, 54, 5747.
doi: 10.1021/jm200394x pmid: 24793885 |
|
|
(j) Galal, S. A.; Khairat, S. H. M.; Ragab, F. A. F.; Abdelsamie, A. S.; Ali, M. M.; Soliman, S. M.; Mortier, J.; Wolber, G.; El Diwani, H. I. Eur. J. Med. Chem. 2014, 86, 122.
doi: 10.1016/j.ejmech.2014.08.048 pmid: 24793885 |
|
|
(k) Hussain, S.; Parveen, S.; Hao, X.; Zhang, S.; Wang, W.; Qin, X.; Yang, Y.; Chen, X.; Zhu, S.; Zhu, C.; Ma, B. Eur. J. Med. Chem. 2014, 80, 383.
doi: 10.1016/j.ejmech.2014.04.047 pmid: 24793885 |
|
|
(l) Khattab, S. N.; Abdel Moneim, S. A. H.; Bekhit, A. A.; El Massry, A. M.; Hassan, S. Y.; El-Faham, A.; Ali Ahmed, H. E.; Amer, A. Eur. J. Med. Chem. 2015, 93, 308.
doi: 10.1016/j.ejmech.2015.02.020 pmid: 24793885 |
|
|
(m) Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; Zhu, C. J. Med. Chem. 2015, 58, 1254.
doi: 10.1021/jm501484b pmid: 24793885 |
|
|
(n) Cil, O.; Phuan, P.-W.; Lee, S.; Tan, J.; Haggie, P. M.; Levin, M. H.; Sun, L.; Thiagarajah, J. R.; Ma, T.; Verkman, A. S. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 317.
doi: 10.1016/j.jcmgh.2015.12.010 pmid: 24793885 |
|
|
(o) Shi, L.; Li, X.; Hu, W.; Wu, J.; Zhou, H.; Zhou, H. Mini-Rev. Med. Chem. 2018, 18, 392.
doi: 10.2174/1389557517666171101111134 pmid: 24793885 |
|
| [2] |
Weïwer, M.; Spoonamore, J.; Wei, J.; Guichard, B.; Ross, N. T.; Masson, K.; Silkworth, W.; Dandapani, S.; Palmer, M.; Scherer, C. A.; Stern, A. M.; Schreiber, S. L.; Munoz, B. ACS Med. Chem. Lett. 2012, 3, 1034.
doi: 10.1021/ml300246r |
| [3] |
(a) Smits, R. A.; Lim, H. D.; Hanzer, A.; Zuiderveld, O. P.; Guaita, E.; Adami, M.; Coruzzi, G.; Leurs, R.; de Esch, I. J. P. J. Med. Chem. 2008, 51, 2457.
doi: 10.1021/jm7014217 pmid: 22639721 |
|
(b) Abu-Hashem, A. A.; Gouda, M. A.; Badria, F. A. Eur. J. Med. Chem. 2010, 45, 1976.
doi: 10.1016/j.ejmech.2010.01.042 pmid: 22639721 |
|
|
(c) Mangold, S. L.; Prost, L. R.; Kiessling, L. L. Chem. Sci. 2012, 3, 772.
pmid: 22639721 |
|
| [4] |
Quinn, J.; Guo, C.; Ko, L.; Sun, B.; He, Y.; Li, Y. RSC Adv. 2016, 6, 22043.
doi: 10.1039/C5RA26227E |
| [5] |
(a) Xie, L.-Y.; Bai, Y.-S.; Xu, X.-Q.; Peng, X.; Tang, H.-S.; Huang, Y.; Lin, Y.-W.; Cao, Z.; He, W.-M. Green Chem. 2020, 22, 1720.
doi: 10.1039/C9GC03899J |
|
(b) Wang, L.; Bao, P.; Liu, W.; Liu, S.; Hu, C.; Yue, H.; Yang, D.; Wei, W. Chin. J. Org. Chem. 2018, 38, 3189. (in Chinese)
doi: 10.6023/cjoc201807014 |
|
|
( 王雷雷, 鲍鹏丽, 刘维伟, 刘思彤, 胡昌松, 岳会兰, 杨道山, 魏伟, 有机化学, 2018, 38, 3189.)
doi: 10.6023/cjoc201807014 |
|
|
(c) Wu, Y.; Chen, J.-Y.; Ning, J.; Jiang, X.; Deng, J.; Deng, Y.; Xu, R.; He, W.-M. Green Chem. 2021, 23, 3950.
doi: 10.1039/D1GC00562F |
|
|
(d) Shi, J.; Wei, W. Chin. J. Org. Chem. 2020, 40, 2170. (in Chinese)
doi: 10.6023/cjoc202000041 |
|
|
( 时建伟, 魏伟, 有机化学, 2020, 40, 2170.)
doi: 10.6023/cjoc202000041 |
|
|
(e) Mao, P.; Zhu, J.; Yuan, J.; Yang, L.; Xiao, Y.; Zhang, C. Chin. J. Org. Chem. 2019, 39, 1529. (in Chinese)
doi: 10.6023/cjoc201904025 |
|
|
( 毛璞, 朱军亮, 袁金伟, 杨亮茹, 肖咏梅, 张长森, 有机化学, 2019, 39, 1529.)
doi: 10.6023/cjoc201904025 |
|
|
(f) Xie, L.-Y.; Peng, S.; Yang, L.-H.; Peng, C.; Lin, Y.-W.; Yu, X.; Cao, Z.; Peng, Y.-Y.; He, W.-M. Green Chem. 2021, 23, 374.
doi: 10.1039/D0GC02844D |
|
|
(g) Yi, R.; He, W. Chin. J. Org. Chem. 2021, 41, 1267. (in Chinese)
doi: 10.6023/cjoc202100022 |
|
|
( 易荣楠, 何卫民, 有机化学, 2021, 41, 1267.)
doi: 10.6023/cjoc202100022 |
|
|
(h) Xie, L.-Y.; Liu, Y.-S.; Ding, H.-R.; Gong, S.-F.; Tan, J.-X.; He, J.-Y.; Cao, Z.; He, W.-M. Chin. J. Catal. 2020, 41, 1168.
doi: 10.1016/S1872-2067(19)63526-6 |
|
|
(i) Chen, J.-Y.; Wu, H.-Y.; Gui, Q.-W.; Yan, S.-S.; Deng, J.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. J. Catal. 2021, 42, 1445.
doi: 10.1016/S1872-2067(20)63750-0 |
|
|
(j) Ke, Q.; Yan, G.; Yu, J.; Wu, X. Org. Biomol. Chem. 2019, 17, 5863.
doi: 10.1039/C9OB00782B |
|
|
(k) Rostoll-Berenguer, J.; Blay, G.; Pedro, J. R.; Vila, C. Eur. J. Org. Chem. 2020, 2020, 6148.
doi: 10.1002/ejoc.v2020.39 |
|
|
(l) Monika, M.; Selvakumar, S. Synthesis 2019, 51, 4113.
doi: 10.1055/s-0037-1611910 |
|
|
(m) Ghosh, P.; Das, S. Synth. Commun. 2020, 50, 2266.
doi: 10.1080/00397911.2020.1765257 |
|
| [6] |
(a) Frontana-Uribe, B. A.; Little, R. D.; Ibanez, J. G.; Palma, A.; Vasquez-Medrano, R. Green Chem. 2010, 12, 2099.
doi: 10.1039/c0gc00382d |
|
(b) Jiang, Y.; Xu, K.; Zeng, C. Chem. Rev. 2018, 118, 4485.
doi: 10.1021/acs.chemrev.7b00271 |
|
|
(c) Jiao, K.-J.; Xing, Y.-K.; Yang, Q.-L.; Qiu, H.; Mei, T.-S. Acc. Chem. Res. 2020, 53, 300.
doi: 10.1021/acs.accounts.9b00603 |
|
|
(d) Karkas, M. D. Chem. Soc. Rev. 2018, 47, 5786.
doi: 10.1039/C7CS00619E |
|
|
(e) Ma, C.; Fang, P.; Mei, T.-S. ACS Catal. 2018, 8, 7179.
doi: 10.1021/acscatal.8b01697 |
|
|
(f) Maeda, H.; Ohmori, H. Acc. Chem. Res. 1999, 32, 72.
doi: 10.1021/ar970329y |
|
|
(g) Minteer, S. D.; Baran, P. Acc. Chem. Res. 2020, 53, 545.
doi: 10.1021/acs.accounts.0c00049 |
|
|
(h) Möhle, S.; Zirbes, M.; Rodrigo, E.; Gieshoff, T.; Wiebe, A.; Waldvogel, S. R. Angew. Chem., nt. Ed. 2018, 57, 6018.
|
|
|
(i) Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S. Chem. Soc. Rev. 2021, 50, 7941.
doi: 10.1039/D1CS00223F |
|
|
(j) Röckl, J. L.; Pollok, D.; Franke, R.; Waldvogel, S. R. Acc. Chem. Res. 2020, 53, 45.
doi: 10.1021/acs.accounts.9b00511 |
|
|
(k) Sauermann, N.; Meyer, T. H.; Qiu, Y.; Ackermann, L. ACS Catal. 2018, 8, 7086.
doi: 10.1021/acscatal.8b01682 |
|
|
(l) Waldvogel, S. R.; Lips, S.; Selt, M.; Riehl, B.; Kampf, C. J. Chem. Rev. 2018, 118, 6706.
doi: 10.1021/acs.chemrev.8b00233 |
|
|
(m) Wang, H.; Gao, X.; Lv, Z.; Abdelilah, T.; Lei, A. Chem. Rev. 2019, 119, 6769.
doi: 10.1021/acs.chemrev.9b00045 |
|
|
(n) Yamamoto, K.; Kuriyama, M.; Onomura, O. Acc. Chem. Res. 2020, 53, 105.
doi: 10.1021/acs.accounts.9b00513 |
|
|
(o) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230.
doi: 10.1021/acs.chemrev.7b00397 |
|
|
(p) Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A. K.; Lei, A. Chem. Rev. 2017, 117, 9016.
doi: 10.1021/acs.chemrev.6b00620 |
|
|
(q) Yuan, Y.; Lei, A. Acc. Chem. Res. 2019, 52, 3309.
doi: 10.1021/acs.accounts.9b00512 |
|
| [7] |
Tan, Y.; Wang, J.; Zhang, H.-Y.; Zhang, Y.; Zhao, J. Front. Chem. 2020, 8, 582.
doi: 10.3389/fchem.2020.00582 |
| [8] |
Lian, F.; Xu, K.; Meng, W.; Zhang, H.; Tan, Z.; Zeng, C. Chem. Commun. 2019, 55, 14685.
doi: 10.1039/C9CC07840A |
| [9] |
Niu, K.; Song, L.; Hao, Y.; Liu, Y.; Wang, Q. Chem. Commun. 2020, 56, 11673.
doi: 10.1039/D0CC05391K |
| [10] |
Traister, K. M.; Molander, G. A. Synthesis and Application of Organoboron Compounds, In Topics in Organometallic Chemistry, Vol. 49, Eds.: Fernández, E.; Whiting, A., Springer, Switzerland, 2015, pp. 117-151.
|
| [11] |
Niu, K.; Hao, Y.; Song, L.; Liu, Y.; Wang, Q. Green Chem. 2021, 23, 302.
doi: 10.1039/D0GC03892J |
| [12] |
Gao, Y.; Wu, Z.; Yu, L.; Wang, Y.; Pan, Y. Angew. Chem., nt. Ed. 2020, 59, 10859.
|
| [13] |
Mo, F.; Qiu, D.; Zhang, L.; Wang, J. Chem. Rev. 2021, 121, 5741.
doi: 10.1021/acs.chemrev.0c01030 |
| [14] |
Jiang, Y.-Y.; Dou, G.-Y.; Zhang, L.-S.; Xu, K.; Little, R. D.; Zeng, C.-C. Adv. Synth. Catal. 2019, 361, 5170.
doi: 10.1002/adsc.v361.22 |
| [15] |
Hu, C.; Hong, G.; Zhou, C.; Tang, Z.-C.; Han, J.-W.; Wang, L.-M. Asian J. Org. Chem. 2019, 8, 2092.
doi: 10.1002/ajoc.v8.11 |
| [16] |
(a) Lehnherr, D.; Lam, Y.-H.; Nicastri, M. C.; Liu, J.; Newman, J. A.; Regalado, E. L.; DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2020, 142, 468.
doi: 10.1021/jacs.9b10870 pmid: 33861088 |
|
(b) Nicastri, M. C.; Lehnherr, D.; Lam, Y.-H.; DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2020, 142, 987.
doi: 10.1021/jacs.9b10871 pmid: 33861088 |
|
|
(c) Zhang, S.; Li, L.; Li, X.; Zhang, J.; Xu, K.; Li, G.; Findlater, M. Org. Lett. 2020, 22, 3570.
doi: 10.1021/acs.orglett.0c01014 pmid: 33861088 |
|
|
(d) Zhang, X.; Yang, C.; Gao, H.; Wang, L.; Guo, L.; Xia, W. Org. Lett. 2021, 23, 3472.
doi: 10.1021/acs.orglett.1c00920 pmid: 33861088 |
|
| [17] |
Wen, J.; Yang, X.; Yan, K.; Qin, H.; Ma, J.; Sun, X.; Yang, J.; Wang, H. Org. Lett. 2021, 23, 1081.
doi: 10.1021/acs.orglett.0c04296 |
| [18] |
(a) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C pmid: 21456523 |
|
(b) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455.
doi: 10.1021/cr100166a pmid: 21456523 |
|
|
(c) Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475.
doi: 10.1021/cr1004293 pmid: 21456523 |
|
|
(d) Liu, T.; Shen, Q. Eur. J. Org. Chem. 2012, 2012, 6679.
doi: 10.1002/ejoc.v2012.34 pmid: 21456523 |
|
| [19] |
Dou, G.-Y.; Jiang, Y.-Y.; Xu, K.; Zeng, C.-C. Org. Chem. Front. 2019, 6, 2392.
doi: 10.1039/C9QO00552H |
| [20] |
(a) Scheuer, P. J. Acc. Chem. Res. 1992, 25, 433.
doi: 10.1021/ar00022a001 pmid: 20804202 |
|
(b) Fleming, F. Nat. Prod. Rep. 1999, 16, 597.
doi: 10.1039/a804370a pmid: 20804202 |
|
|
(c) Fleming, F. F.; Yao, L.; Ravikumar, P. C.; Funk, L.; Shook, B. C. J. Med. Chem. 2010, 53, 7902.
doi: 10.1021/jm100762r pmid: 20804202 |
|
| [21] |
(a) Larock, R. C. Comprehensive Organic Transformations, Wiley-VCH, New York, 1989, p. 819.
|
|
(b) Caboni, P.; Sammelson, R. E.; Casida, J. E. J. Agric. Food Chem. 2003, 51, 7055.
doi: 10.1021/jf030439l |
|
|
(c) Kuhn, P.; Thomas, A.; Antonietti, M. Macromolecules 2009, 42, 319.
doi: 10.1021/ma802322j |
|
| [22] |
(a) Liskey, C. W.; Liao, X.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 11389.
doi: 10.1021/ja104442v pmid: 20677758 |
|
(b) Wade, J. R. Organic Chemistry, 8 th ed., Pearson Education Inc, Glenview, Illinois, USA, 2013.
pmid: 20677758 |
|
|
(c) Zhang, S.; del Pozo, J.; Romiti, F.; Mu, Y.; Torker, S.; Hoveyda, A. H. Science 2019, 364, 45.
doi: 10.1126/science.aaw4029 pmid: 20677758 |
|
| [23] |
Zhan, Y.; Li, Y.; Tong, J.; Liu, P.; Sun, P. Eur. J. Org. Chem. 2021, 2021, 2193.
|
| [24] |
(a) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257.
doi: 10.1021/jm501100b pmid: 26507237 |
|
(b) Cernak, T.; Dykstra, K. D.; Tyagarajan, S.; Vachal, P.; Krska, S. W. Chem. Soc. Rev. 2016, 45, 546.
doi: 10.1039/c5cs00628g pmid: 26507237 |
|
| [25] |
Li, K.-J.; Xu, K.; Liu, Y.-G.; Zeng, C.-C.; Sun, B.-G. Adv. Synth. Catal. 2019, 361, 1033.
doi: 10.1002/adsc.v361.5 |
| [26] |
Jiang, X.; Yang, L.; Ye, Z.; Du, X.; Fang, L.; Zhu, Y.; Chen, K.; Li, J.; Yu, C. Eur. J. Org. Chem. 2020, 2020, 1687.
doi: 10.1002/ejoc.201901928 |
| [27] |
Song, C.; Liu, K.; Dong, X.; Chiang, C.-W.; Lei, A. Synlett 2019, 30, 1149.
doi: 10.1055/s-0037-1611753 |
| [28] |
Zhou, J.; Li, Z.; Sun, Z.; Ren, Q.; Zhang, Q.; Li, H.; Li, J. J. Org. Chem. 2020, 85, 4365.
doi: 10.1021/acs.joc.0c00050 |
| [29] |
(a) Demmer, C. S.; Krogsgaard-Larsen, N.; Bunch, L. Chem. Rev. 2011, 111, 7981.
doi: 10.1021/cr2002646 pmid: 30300843 |
|
(b) Montchamp, J.-L. Acc. Chem. Res. 2014, 47, 77.
doi: 10.1021/ar400071v pmid: 30300843 |
|
|
(c) Mady, M. F.; Kelland, M. A. Energy Fuels 2017, 31, 4603.
doi: 10.1021/acs.energyfuels.7b00708 pmid: 30300843 |
|
|
(d) Ntatsopoulos, V.; Macegoniuk, K.; Mucha, A.; Vassiliou, S.; Berlicki, Ł. Eur. J. Med. Chem. 2018, 159, 307.
doi: S0223-5234(18)30859-6 pmid: 30300843 |
|
| [30] |
Li, K.-J.; Jiang, Y.-Y.; Xu, K.; Zeng, C.-C.; Sun, B.-G. Green Chem. 2019, 21, 4412.
doi: 10.1039/C9GC01474H |
| [1] | 徐利军, 李宗军, 韩福社, 高翔. N,N-二甲基甲酰胺促进的富勒烯稠合噁唑啉衍生物的合成[J]. 有机化学, 2024, 44(1): 242-250. |
| [2] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [3] | 钟赟哲, 陈颖, 俞磊, 周宏伟. 电化学介导羧酸与醇的酯化反应[J]. 有机化学, 2023, 43(8): 2855-2863. |
| [4] | 张周, 郭钰, 羊静, 吴丹, 王佳昕, 洪欣玥, 蔡佩君, 荣良策. 电化学促进咪唑并[1,2-a]吡啶与二氯(溴)乙烷及碘仿的卤化反应[J]. 有机化学, 2023, 43(6): 2104-2109. |
| [5] | 张俊颖, 赵晓静, 李干鹏, 何永辉. 室温下电化学合成保护型有机硼酸RB(dan)[J]. 有机化学, 2023, 43(5): 1815-1823. |
| [6] | 杜琳琳, 张华. 芳烃与烷烃化合物参与的光化学与电化学硼化反应[J]. 有机化学, 2023, 43(5): 1726-1741. |
| [7] | 孙丽, 宋国欣, 韩家乐, 李继玉, 赵月, 杨璐华, 张峰, 赵坤, 毛比明. Morita-Baylis-Hillman加合物和N-羟基邻苯二甲酰亚胺的电化学烯丙基烷基化形成C(sp3)—C(sp3)键[J]. 有机化学, 2023, 43(4): 1574-1583. |
| [8] | 潘永周, 蒙秀金, 王迎春, 何慕雪. 电化学固定CO2构建羧酸衍生物的研究进展[J]. 有机化学, 2023, 43(4): 1416-1434. |
| [9] | 黄嘉为, 李潇漫, 徐亮, 韦玉. α-酮酸与硫酚的电化学脱羧偶联: 一种合成硫代酸酯的新方法[J]. 有机化学, 2023, 43(2): 756-762. |
| [10] | 危斌, 周子龙, 秦景灏, 严泽宇, 郭嘉程, 雷澍, 谢叶香, 欧阳旋慧, 宋仁杰. 氧杂蒽与亚磺酸钠的电化学氧化C(sp3)—H磺酰化反应[J]. 有机化学, 2023, 43(1): 186-194. |
| [11] | 魏琬絜, 詹磊, 高雷, 黄国保, 马献力. 电化学合成C-磺酰基化合物的研究进展[J]. 有机化学, 2023, 43(1): 17-35. |
| [12] | 李海琼, 尹梦云, 谢芬芬, 张正兵, 韩盼, 敬林海. 通过电化学Appel反应合成腈[J]. 有机化学, 2022, 42(7): 2229-2235. |
| [13] | 顾清云, 程振凤, 曾小宝. 电化学氧化三氟甲基亚磺酸钠与α-羰基二硫缩烯酮的三氟甲基化反应[J]. 有机化学, 2022, 42(5): 1537-1544. |
| [14] | 付拯江, 杨振江, 孙丽, 尹健, 伊学政, 蔡琥, 雷爱文. 无金属条件下亚磺酸钠与酚类化合物形成芳基磺酸酯的电化学合成反应[J]. 有机化学, 2022, 42(2): 600-606. |
| [15] | 锅小龙, 王玉贤, 赵志强, 王庆, 左剑, 王陆瑶. 电化学氧化下喹喔啉-2(1H)-酮的三氟甲基化及电描述符对反应性能的评价[J]. 有机化学, 2022, 42(2): 641-649. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||