有机化学 ›› 2025, Vol. 45 ›› Issue (4): 1206-1222.DOI: 10.6023/cjoc202407012 上一篇 下一篇
综述与进展
收稿日期:2024-07-04
修回日期:2024-09-01
发布日期:2024-10-10
基金资助:
Jia Zhaoa( ), Qiuyun Gana, Yaofeng Yuanb(
), Qiuyun Gana, Yaofeng Yuanb( )
)
Received:2024-07-04
Revised:2024-09-01
Published:2024-10-10
Contact:
* E-mail: Supported by:文章分享

磺酰氟作为有机合成中具有独特反应活性的官能团, 已经在药物合成、化学生物学和材料科学等多个领域展现出巨大的应用潜力. 然而, 随着现代合成化学对于环境友好性和可持续性要求的不断提高, 传统的磺酰氟合成方法面临着越来越大的挑战. 与传统方法相比, 自由基磺酰氟化反应因其反应高效、能耗低和环境友好的特点, 逐渐成为引入磺酰氟基团的重要手段, 在有机合成中发挥出越来越重要的作用. 此综述将重点介绍自由基磺酰氟化反应方面的最新进展.
赵佳, 甘秋云, 袁耀锋. 自由基磺酰氟化反应研究进展[J]. 有机化学, 2025, 45(4): 1206-1222.
Jia Zhao, Qiuyun Gan, Yaofeng Yuan. Research Progress in Free Radical Fluorosulfonylation[J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1206-1222.
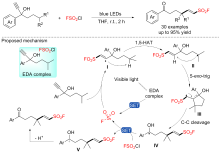
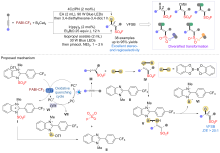

| [1] |
Dong, J.-J.; Krasnova, L.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2014, 53, 9430.
|
| [2] |
Akamanchi, K.; Bellale, E.; Chaudhari, M. Synthesis 2009, 2009, 3211.
|
| [3] |
Wray, K. L.; Feldman, E. V. J. Chem. Phys. 1971, 54, 3445.
|
| [4] |
Kiang, T.; Zare, R. N. J. Chem. Soc., Chem. Commun. 1980, 24, 1228.
|
| [5] |
Leusen, V.; Wildeman, A. M.; Oldenziel, J. O. H. J. Org. Chem. 1977, 42, 1153.
|
| [6] |
Frye, L. L.; Sullivan, E. L.; Cusack, K. P. J. Org. Chem. 1992, 57, 697.
|
| [7] |
Truce, W. E.; Madding, G. D. Tetrahedron Lett. 1966, 7, 3681.
|
| [8] |
Bashir, R.; Awadi, F.; N. A.; Dusouqui, O. M. E. Can. J. Chem. 2005, 83, 1543.
|
| [9] |
Horner, L.; Göwecke, S. Chem. Ber. 1961, 94, 1267.
|
| [10] |
Hyatt, J. A.; White, A. W. Synthesis 1984, 1984, 214.
|
| [11] |
Giel, M. C.; Smedley, C. J.; Mackie, E. R. R.; Guo, T.-J.; Dong, J.-J.; Soares da Costa, T. P.; Moses, J. E. Angew. Chem., Int. Ed. 2020, 59, 1181.
|
| [12] |
Liang, D.-D.; Streefkerk, D. E.; Jordaan, D.; Wagemakers, J.; Baggerman, J.; Zuilhof, H. Angew. Chem., Int. Ed. 2020, 59, 7494.
|
| [13] |
Jones, L. H. ACS Med. Chem. Lett. 2018, 9, 584.
doi: 10.1021/acsmedchemlett.8b00276 pmid: 30034581 |
| [14] |
Baggio, C.; Udompholkul, P.; Gambini, P.; Salem, F.; Jossart, J.; Perry, J. J. P.; Pellecchia, M. J. Med. Chem. 2019, 62, 9188.
doi: 10.1021/acs.jmedchem.9b01108 pmid: 31550155 |
| [15] |
Galvin, C. J.; Genzer, J. Prog. Polym. Sci. 2012, 37, 871.
|
| [16] |
Xiao, X.; Zhou, F.; Jiang, J.; Chen, H.-F.; Wang, L.-H.; Chen, D.-Y.; Xu, Q.-F.; Lu, J.-M. Polym. Chem. 2018, 9, 1040.
|
| [17] |
Durie, K.; Yatvin, J.; Kovaliov, M.; Crane, G. H.; Horn, J.; Averick, S.; Locklin, J. Macromolecules 2018, 51, 297.
|
| [18] |
Chao, Y.; Krishna, A.; Subramaniam, M.; Liang, D.-D.; Pujari, S. P.; Sue, A. C.-H.; Li, G.; Miloserdov, F. M.; Zuilhof, H. Angew. Chem., Int. Ed. 2022, 61, e202207456.
|
| [19] |
Wang, M.; Jin, H.-S.; Chen, X.-M.; Lin, B.-P.; Yang, H. Polym. Chem. 2019, 10, 3657.
|
| [20] |
Wan, H.-B.; Zhou, S.-Y.; Gu, P.-Y.; Zhou, F.; Lyu, D.; Xu, Q.-H.; Wang, A.-N.; Shi, H.-B.; Xu, Q.-F.; Lu, J.-M. Polym. Chem. 2020, 11, 1033.
|
| [21] |
Baker, B. R.; Hurlbut, J. A. J. Med. Chem. 1969, 12, 221.
pmid: 5783594 |
| [22] |
Teng, M.-X.; Ficarro, S. B.; Yoon, H. J.; Che, J.-W.; Zhou, J.; Fischer, E. S.; Marto, J. A.; Zhang, T.-H.; Gray, N. S. ACS Med. Chem. Lett. 2020, 11, 1269.
|
| [23] |
Bashashati, M.; Storr, M.; Nikas, S.; Wood, J.; Godlewski, G.; Liu, J.; Ho, W.; Keenan, C.; Zhang, H.; Alapafuja, S.; Cravatt, B.; Lutz, B.; Mackie, K.; Kunos, G.; Patel, K.; Makriyannis, A.; Davison, J.; Sharkey, K. Br. J. Pharmacol. 2012, 165, 1556.
|
| [24] |
Kitamura, S.; Zheng, Q.-H.; Woehl, J. L.; Solania, A.; Chen, E.; Dillon, N.; Hull, M. V.; Kotaniguchi, M.; Cappiello, J. R.; Kitamura, S.; Nizet, V.; Sharpless, K. B.; Wolan, D. W. J. Am. Chem. Soc. 2020, 142, 10899.
doi: 10.1021/jacs.9b13652 pmid: 32479075 |
| [25] |
Bianchi, T. A.; Cate, L. A. J. Org. Chem. 1977, 42, 2031.
|
| [26] |
Wang, L.; Cornella, J. Angew. Chem., Int. Ed. 2020, 59, 23510.
|
| [27] |
Wright, S. W.; Hallstrom, K. N. J. Org. Chem. 2006, 71, 1080.
|
| [28] |
Zhang, L.; Cheng, X.; Zhou, Q.-L. Chin. J. Chem. 2022, 40, 1687.
|
| [29] |
Laudadio, G.; Bartolomeu, A. Verwijlen, L. M. H. M.; Cao, Y.-R.; de Oliveira, K. T.; Noël, T. J. Am. Chem. Soc. 2019, 141, 11832.
|
| [30] |
Shavnya, A.; Coffey, S. B.; Hesp, K. D.; Ross, S. C.; Tsai, A. S. Org. Lett. 2016, 18, 5848.
pmid: 27788009 |
| [31] |
Shavnya, A.; Hesp, K. D.; Tsai, A. S. Adv. Synth. Catal. 2018, 360, 1768.
|
| [32] |
Liu, Y.-A.; Yu, D.-H.; Guo, Y.; Xiao, J.-C.; Chen, Q.-Y.; Liu, C. Org. Lett. 2020, 22, 2281.
|
| [33] |
Zhong, T.; Yi, J.; Chen, Z.; Zhuang, Q.-L.; Lu, Y.; Weng, G. J. Chem. Sci. 2021, 7, 9359.
|
| [34] |
Sarver, P. J.; Bissonnette, N. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2021, 143, 9737.
|
| [35] |
Ma, Z.; Liu, Y.; Ma, X.; Hu, X.; Guo, Y.; Chen, Q.; Liu, Y.-C. Org. Chem. Front. 2022, 9, 1115.
|
| [36] |
Nguyen, V. T.; Haug, G. C.; Nguyen, V. D.; Vuong, N. T. H.; Karki, G. B.; Arman, H. D.; Larionov, O. V. Chem. Sci. 2022, 13, 4170.
doi: 10.1039/d2sc00789d pmid: 35440976 |
| [37] |
McDowell, C. A.; Herring, F. G.; Tait, J. C. J. Chem. Phys. 1975, 63, 3278.
|
| [38] |
Boyd, R. J.; Gupta, A.; Langler, R. F. Can. J. Chem. 1980, 58, 331.
|
| [39] |
Li, Z.-J. Chem. Phys. Lett. 1997, 269, 128.
|
| [40] |
Zeng, X.-Q.; Beckers, H.; Willner, H. J. Am. Chem. Soc. 2013, 135, 2096.
|
| [41] |
Zhong, T.; Chen, Z.-D.; Yi, J.-T.; L, G.; Weng, J. Chin. Chem. Lett. 2021, 32, 2736.
|
| [42] |
Lee, C.; Cook, A. J.; Elisabeth, E. J.; Friede, N. C.; Sammis, G. M.; Ball, N. D. ACS Catal. 2021, 11, 6578.
|
| [43] |
Chelagha, A.; Louvel, D.; Taponard, A.; Berthelon, R.; Tlili, A. Catalysts 2021, 11, 830.
|
| [44] |
Lou, T. S. B.; Willis, M. C. Nat. Rev. Chem. 2022, 6, 146.
|
| [45] |
He, F.-S.; Li, Y.-Q.; Wu, J. Org. Chem. Front. 2022, 9, 5299.
|
| [46] |
Barata-Vallejo, S.; Yerien, D. E.; Postigo, A. Catal. Sci. Technol. 2023, 13, 2597.
|
| [47] |
Zheng, Y.; Lu, W.-G.; Ma, T.-T.; Huang, S.-L. Org. Chem. Front. 2024, 11, 217.
|
| [48] |
Lin, L.; Pei, G.-H.; Cao, Z.-Y.; Liao, S.-H. Eur. J. Org. Chem. 2024, e202400279.
|
| [49] |
Van Dyke Tiers, G. US 2846472, 1956.
|
| [50] |
Nie, X.-L.; Xu, T.-X.; Song, J.-S.; Devaraj, A.; Zhang, B.-L.; Chen, Y.; Liao, S.-H. Angew. Chem., Int. Ed. 2021, 60, 3956.
|
| [51] |
Nie, X.-L.; Xu, T.-X.; Hong, Y.-H.; Zhang, H.-H.; Mao, C.-X.; Liao, S.-H. Angew. Chem., Int. Ed. 2021, 60, 22035.
|
| [52] |
Zhao, X.-Y.; Chen, D.-F.; Zhu, S.-Z.; Luo, J.-Y.; Liao, S.-H.; Zheng, B.-N.; Huang, S.-L. Org. Lett. 2023, 25, 3109.
|
| [53] |
Shi, S.; Zhao, X.-Y.; Chen, D.-F.; Luo, J.-Y.; Liao, S.-H.; Huang, S.-L. Org. Chem. Front. 2023, 10, 3805.
|
| [54] |
He, T.-Y.; Liang, C.-Q.; Jiang, P.; Liang, H.; Liao, S.-H.; Huang, S.-L. Org. Lett. 2024, 26, 5577.
|
| [55] |
Zhang, Y.-Y.; Feng, Q.-Y.; Zheng, Y.; Lu, Y.-J.; Liao, S.-H.; Huang, S.-L. Org. Lett. 2024, 26, 1410.
|
| [56] |
Cheng, H.-Y.; He, T.-Y.; Chen, D.-F.; Zheng, Y.; Lu, Y.; Liang, H.; Liao, S.-H.; Huang, S.-L. Org. Lett. 2024, 26, 3581.
|
| [57] |
Chen, D.-F.; Nie, X.-L.; Feng, Q.-Y.; Zhang, Y.-Y.; Wang, Y.-H.; Wang, Q.-Y.; Huang, L.; Huang, S.-L.; Liao, S.-H. Angew. Chem., Int. Ed. 2021, 60, 27271.
|
| [58] |
Feng, Q.-Y.; Fu, Y.-Y.; Zheng, Y.; Liao, S.-H.; Huang, S.-L. Org. Lett. 2022, 24, 3702.
|
| [59] |
Feng, Q.-Y.; He, T.-Y.; Qian, S.-C.; Xu, P.; Liao, S.-H.; Huang, S.-L. Nat. Commun. 2023, 14, 8278.
|
| [60] |
Guo, T.-J.; Meng, G.-Y.; Zhan, X.-J.; Yang, Q.; Ma, T.-C.; Xu, L.; Sharpless, K. B.; Dong, J.-J. Angew. Chem., nt. Ed. 2018, 57, 2605.
|
| [61] |
Wang, P.; Zhang, H.-H.; Nie, X.-L.; Xu, T.-X.; Liao, S.-H. Nat. Commun. 2022, 13, 3370.
|
| [62] |
Zhang, W.-G.; Li, H.-Y.; Li, X.-J.; Zou, Z.-L.; Huang, M.-J.; Liu, J.-Y.; Wang, X.-C.; Ni, S.-Y.; Pan, Y.; Wang, Y. Nat. Commun. 2022, 13, 3515.
|
| [63] |
Wang, P.; Zhang, H.-H.; Zhao, M.-Q.; Ji, S.-S.; Lin, L.; Yang, N.; Nie, X.-L.; Song, J.-S.; Liao, S.-H. Angew. Chem., Int. Ed. 2022, 61, e202207684.
|
| [64] |
Zhang, H.-H.; Yang, N.; Li, J.; Wang, P.; Li, S.-J.; Xie, L.-L.; Liao, S.-H. Org. Lett. 2022, 24, 8170.
|
| [65] |
Lin, L.; Wang, P.; Dong, T.; Tsui, G. C.; Liao, S.-H. Org. Lett. 2023, 25, 1088.
doi: 10.1021/acs.orglett.2c04315 pmid: 36775923 |
| [66] |
Yang, N.; Mao, C.-X.; Zhang, H.-H.; Wang, P.; Li, S.-J.; Xie, L.-L.; Liao, S.-H. Org. Lett. 2023, 25, 4478.
doi: 10.1021/acs.orglett.3c01491 pmid: 37306334 |
| [67] |
Li, H.-Y.; Huang, M.-J.; Zou, Z.-L.; Wang, Z.; Li, Y.-F.; Sun, C.; Chen, W.-Z.; Pan, Y.; Zhang, W.-G.; Wang, Y. Chem. Sci. 2023, 14, 13893.
|
| [68] |
Fang, X.; Geng, X.-B.; Wang, P.; Zhang, H.-H.; Liao, S.-H. Synthesis 2024, 56, 1727.
|
| [69] |
Dong, X.-R.; Jiang, W.-H.; Hua, D.-X.; Wang, X.-H.; Xu, L.; Wu, X.-X. Chem. Sci. 2021, 12, 11762.
|
| [70] |
Frye, N. L.; Daniliuc, C. G.; Studer, A. Angew. Chem., Int. Ed. 2022, 61, e202115593.
|
| [71] |
Erchinger, J. E.; Hoogesteger, R.; Laskar, R.; Dutta, S.; Hümpel, C.; Rana, D.; Daniliuc, C. G.; Glorius, F. J. Am. Chem. Soc. 2023, 145, 2364.
|
| [72] |
Cui, J.-C.; Ke, S.; Zhao, J.; Wu, S.-S.; Luo, W.-C.; Xu, S.-N.; Su, X.-L.; Li, Y. Org. Chem. Front. 2022, 9, 3540.
|
| [73] |
Xiong, T.; Chen, Q.-L.; Chen, Z.-D.; Lu, G.; Chan, A. S. C.; Weng, J. Chem. Catal. 2023, 3, 100821.
|
| [1] | 韩哲, 崔银, 丁成荣, 杨建, 张国富. “SO2F”源直接构建磺酰氟官能团的合成应用研究进展[J]. 有机化学, 2025, 45(7): 2245-2264. |
| [2] | 张放, 谢佩, 张启超, 武磊芳, 何林, 杜广芬. Brønsted碱促进的o-羟基席夫碱与乙烯基磺酰氟通过Michael-Mannich-SuFEx串联反应构建磺基色烯并[4,3‑b]吡咯烷化合物[J]. 有机化学, 2025, 45(7): 2552-2565. |
| [3] | 谭永波, 舒洪波, 黄华文. 光诱导N-芳基丙烯酰胺参与的吲哚酮合成研究进展[J]. 有机化学, 2025, 45(6): 2086-2108. |
| [4] | 姚嫣, 付年凯. 光电化学金属催化研究进展[J]. 有机化学, 2025, 45(6): 1819-1837. |
| [5] | 高根伟, 李震, 李炎, 陆熹. 光/镍协同催化C(sp2)—C(sp3)键构建研究进展[J]. 有机化学, 2025, 45(6): 1905-1919. |
| [6] | 蒋晨阳, 尹艳丽, 江智勇. 光酶催化不对称自由基加成反应研究进展[J]. 有机化学, 2025, 45(5): 1614-1633. |
| [7] | 吴昊岩, 赫明月, 马军安, 张发光. 三芳胺在光催化反应中的研究进展[J]. 有机化学, 2025, 45(5): 1634-1643. |
| [8] | 陈旭欣, 张丹丹, 龙鸿新, 刘国凯. 无需光敏剂光诱导炔丙酸酯自由基二氟甲基化合成3-二氟甲基香豆素[J]. 有机化学, 2025, 45(5): 1739-1746. |
| [9] | 林风, 张艳, 吴明, 刘会艳, 郝文娟, 姜波. 利用可见光引发1,6-烯炔的增环酰化双官能化制备1-茚酮衍生物[J]. 有机化学, 2025, 45(5): 1729-1738. |
| [10] | 洪洋, 邓红平. 可见光催化的酸性C(sp3)—H键官能团化反应研究进展[J]. 有机化学, 2025, 45(5): 1569-1590. |
| [11] | 郭仕勋, 王卫, 张永强. 三线态能量转移促进的自由基链式烯烃氢芳基化反应研究[J]. 有机化学, 2025, 45(5): 1716-1728. |
| [12] | 李晨, 焦毅, 施笑然, 杨毅强, 俞寿云. 光氧化还原催化的C-糖基化反应[J]. 有机化学, 2025, 45(5): 1423-1459. |
| [13] | 谭芳芳, 史孟欣, 张文敏, 李洋. 光催化生物质相关转化[J]. 有机化学, 2025, 45(5): 1523-1547. |
| [14] | 李慧, 阿布力米提•阿布都卡德尔, 周磊. 可见光条件下N-苄基苯并三氮唑自由基脱氮气开环合成6-取代菲啶[J]. 有机化学, 2025, 45(5): 1770-1777. |
| [15] | 李顺曦, 游力栩, 李玉龙, 舒伟. 光介导氨及其等价体参与的碳氮成键反应研究进展[J]. 有机化学, 2025, 45(5): 1460-1477. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||