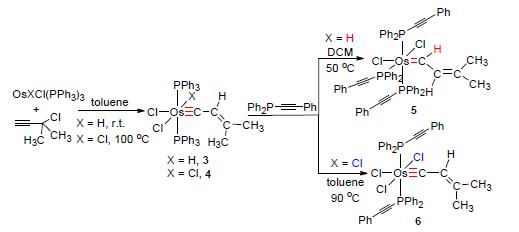
| [1] For selected general reviews on the chemistry of transition metal carbyne complexes see:
(a) Fischer, H.; Hofmann, P.; Kreissl, F. R.; Schrock, R. R.; Schubert, U.; Weiss, K. In Carbyne Complexes, VCH, Weinheim, Germany, 1988.
(b) Maya, A.; Hoffmeister, H. Adv. Organomet. Chem. 1991, 32, 227.
(c) Engel, P. F.; Pfeffer, M. Chem. Rev. 1995, 95, 2281.
(d) Schrock, R. R. J. Chem. Soc., Dalton Trans. 2001, 2541.
(e) Schrock, R. R. Chem. Rev. 2002, 102, 145.
(f) Shi, C.; Jia, G. Coord. Chem. Rev. 2013, 257, 666.
(g) Herndon, J. W. Coord. Chem. Rev. 2016, 317, 1.
[2] For selective reviews on alkyne metathesis see:
(a) Fürstner, A. Angew. Chem., Int. Ed. 2013, 52, 2794 and references cited therein.
(b) Schrock, R. R. Angew. Chem., Int. Ed. 2006, 45, 3748.
(c) Zhang, W.; Moore, J. S. Adv. Synth. Catal. 2007, 349, 93.
(d) Fürstner, A.; Davies, P. W. Chem. Commun. 2005, 2307.
(e) Bunz, H. F. Acc. Chem. Res. 2001, 34, 998.
[3] Selected recent examples of alkyne metathesis:
(a) on Kugelgen, S.; Bellone, D. E.; Cloke, R. R.; Perkins, W. S.; Fischer, F. R. J. Am. Chem. Soc. 2016, 138, 6234.
(b) Wang, Q.; Yu, C.; Long, H.; Du, Y.; Jin, Y.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 7550.
(c) Ralston, K. J.; Ramstadius, H. C.; Brewster, R. C.; Niblock, H. S.; Hulme, A. N. Angew. Chem., Int. Ed. 2015, 54, 7086.
(d) Haberlag, B.; Freytag, M.; Jones, P. G.; Tamm, M. Adv. Synth. Catal. 2014, 356, 1255.
(e) Lhermet, R.; Fürstner, A. Chem. Eur. J. 2014, 20, 13188.
(f) Li, S. T.; Schnabel, T.; Lysenko, S.; Brandhorst, K.; Tamm, M. Chem. Commun. 2013, 49, 7189.
(g) Yang, H.; Jin, Y.; Du, Y.; Zhang, W. J. Mater. Chem. A 2014, 2, 5986.
(h) Heppekausen, J.; Stade, R.; Goddard, R.; Fürstner, A. J. Am. Chem. Soc. 2010, 132, 11045.
(i) Weissman, H.; Plunkett, K. N.; Moore, J. S. Angew. Chem., Int. Ed. 2006, 45, 585.
[4] For reviews on osmium carbyne complexes see:
(a) Bolaño, T.; Esteruelas, M. A.; Oñate, E. J. Organomet. Chem. 2011, 696, 3911.
(b) Jia, G.. Coord. Chem. Rev. 2007, 251, 2167.
(c) Roper, W. R. In Transition Metal Carbyne Complexes, Ed.:Kreissl, F. R., Kluwer Academic:Dordrecht, The Netherlands, 1993, 155.
(d) Gallop, M. A.; Roper, W. R. Adv. Organomet. Chem. 1986, 25, 121.
[5] Selected recent examples of osmium carbyne complexes:
(a) Casanova, N.; Esteruelas, M. A.; Gulías, M.; Larramona, C.; Mascareñas, J. L.; Oñate, E. Organometallics 2016, 35, 91.
(b) Zhou, X.; He, X.; Lin, J.; Zhuo, Q.; Chen, Z.; Zhang, H.; Wang, J.; Xia, H. Organometallics 2015, 34, 1742.
(c) Chen, J.; Huang, Z.-A.; Hua, Y.; Zhang, H.; Xia, H. Organometallics 2015, 34, 340.
(d) Buil, M. L.; Cardo, J. J. F.; Esteruelas, M. A.; Fernández, I.; Oñate, E. Organometallics 2015, 34, 547.
(e) Chen, J.; Zhang, C.; Xie, T.; Wen, T. B.; Zhang, H.; Xia, H. Organometallics 2013, 32, 3993.
(f) An, R.; Li, T.; Wen, T.-B. Chin. J. Org. Chem. 2013, 33, 1697(in Chinese).(安冉, 李亭, 温庭斌, 有机化学, 2013, 33, 1697.)
(g) Chen, J. X.; Sung, H. H. Y.; Williams, I. D.; Jia, G. Organometallics 2011, 30.
(h) Richter, B.; Werner, H. Organometallics 2009, 28, 5137.
(i) Bolaño, T.; Alba Collado, A.; Esteruelas, M. A.; Oñate, E. Organometallics 2009, 28, 2107.
(g) Castro-Rodrigo, R.; Esteruelas, M. A.; López, A. M.; Oñate, E. Organometallics 2008, 27, 3547.
(k) Castarlenas, R.; Esteruelas, M. A.; Lalrempuia, R.; Oliván, M.; Oñate, E. Organometallics 2008, 27, 795.
(l) Lee, J. H.; Pink, M.; Caulton, K. G. Organometallics 2006, 25, 802.
(m) Wen, T. B.; Hung, W. Y.; Zhou, Z. Y.; Lo, M. F.; Williams, I. D.; Jia, G. Eur. J. Inorg. Chem. 2004, 2837.
(n) Asensio, A.; Buil, M. L.; Esteruelas, M. A.; Oñate, E. Organometallics 2004, 23, 5787.
(o) Esteruelas, M. A.; López, A. M.; Oñate, E.; Royo, E. Organometallics 2004, 23, 3021.
(p) Wen, T. B.; Zhou, Z. Y.; Lo, M. F.; Williams, I. D.; Jia, G. Organometallics 2003, 22, 5217.
(q) Barrio, P.; Esteruelas, M. A.; Oñate, E. Organometallics 2003, 22, 2472.
(r) Weberndörfer, B.; Henig, G.; Hockless, D. C. R.; Bennett, M. A.; Werner, H. Organometallics 2003, 22, 744.
(s) Castarlenas, R.; Esteruelas, M. A.; Oñate, E. Organometallics 2001, 20, 3283.
(t) Wen, T. B.; Cheung, Y. K.; Yao, J.; Wong, W. T.; Zhou, Z. Y.; Jia, G. Organometallics 2000, 19, 3803.
[6] (a) Buil, M. L.; Cardo, J. J. F.; Esteruelas, M. A.; Oñate, E. Organometallics 2016, 35, 2171.
(b) Buil, M. L.; Cardo, J. J. F.; Esteruelas, M. A.; Fernández, I.; Oñate, E. Organometallics 2014, 33, 2689.
(c) Bolaño, T.; Castarlenas, R.; Esteruelas, M. A.; Modrego, F. J.; Oñate, E. J. Am. Chem. Soc. 2005, 127, 11184.
(d) Barrio, P.; Esteruelas, M. A.; Oñate, E. J. Am. Chem. Soc. 2004, 126, 1946.
[7] (a) Jia, G. Organometallics 2013, 32, 6852 and references cited therein.
(b) Chen, J.; He, G.; Jia, G. Chin. J. Org. Chem. 2013, 33, 792(in Chinese).(陈江溪, 何国梅, 贾国成, 有机化学, 2013, 33, 792.)
(c) Chen, J.; Shi, C.; Sung, H. H. Y.; Williams, I. D.; Lin, Z.; Jia, G.. Angew. Chem., Int. Ed. 2011, 50, 7295.
(d) Hung, W. Y.; Liu, B.; Shou, W.; Wen, T. B.; Shi, C.; Sung, H. H.-Y.; Williams, I. D.; Lin, Z.; Jia, G. J. Am. Chem. Soc. 2011, 133, 18350.
(e) He, G.; Zhu, J.; Hung, W. Y.; Wen, T. B.; Sung, H. H. Y.; Williams, I. D.; Lin, Z.; Jia, G. Angew. Chem. Int. Ed. 2007, 46, 9065.
(f) Hung, W. Y.; Zhu, J.; Wen, T. B.; Yu, K. P.; Sung, H. H.-Y.; Williams, I. D.; Lin, Z.; Jia, G. J. Am. Chem. Soc. 2006, 128, 13742.
(g) Jia, G. Acc. Chem. Res. 2004, 37, 479.
[8] (a) Cao, X.-Y.; Zhao, Q.; Lin, Z.; Xia, H. Acc. Chem. Res. 2014, 47, 341.
(b) Chen, J.; Huang, Z.-A.; Lu, Z.; Zhang, H.; Xia, H. Chem. Eur. J. 2016, 22, 5363.
(c) Zhu, C.; Yang, Y.; Luo, M.; Yang, C.; Wu, J.; Chen, L.; Liu, G.; Wen, T.; Zhu, J.; Xia, H. Angew. Chem., Int. Ed. 2015, 54, 6181.
(d) Zhu, C.; Li, S.; Luo, M.; Zhou, X.; Niu, Y.; Lin, M.; Zhu, J.; Cao, Z.; Lu, X.; Wen, T.; Xie, Z.; Schleyer, P. V. R.; Xia, H. Nat. Chem. 2013, 5, 698.
(e) Liu, B.; Xie, H.; Wang, H.; Wu, L.; Zhao, Q.; Chen, J.; Wen, T. B.; Cao, Z.; Xia, H. Angew. Chem., Int. Ed. 2009, 48, 5461.
(f) Liu, B.; Wang, H.; Xie, H.; Zeng, B.; Chen, J.; Tao, J.; Wen, T. B.; Cao, Z.; Xia, H. Angew. Chem., Int. Ed. 2009, 48, 5430.
[9] Crabtree, R. H. The Organometallic Chemistry of the Transition Metals, 4th ed., John Wiley & Sons, New York, 2005, pp. 309~340.
[10] (a) Mayr, A.; Asaro, M. F.; Kjelsberg, M. A.; Lee, K. S.; Van Engen, D. Organometallics 1987, 6, 432.
(b) Doyle, R. A.; Angelici, R. J. Organometallics 1989, 8, 2207.
(c) Blosch, L. L.; Abboud, K.; Boncella, J. M. J. Am. Chem. Soc. 1991, 113, 7066.
(d) Bastos, C. M.; Daubenspeck, N.; Mayr, A. Angew. Chem., Int. Ed. Engl. 1993, 32, 743.
(e) Giannini, L.; Solari, E.; Floriani, C.; Chiesi-Villa, A.; Rizzoli, C. J. Am. Chem. Soc. 1998, 120, 823.
(f) Bannwart, E.; Jacobsen, H.; Furno, F.; Berke, H. Organometallics 2000, 19, 3605.
[11] Boone, M. P.; Brown, C. C.; Ancelet, T. A.; Stephan, D. W. Organometallics 2010, 29, 4369.
[12] Esteruelas, M. A.; López, A. M.; Oliván, M. Coord. Chem. Rev. 2007, 251, 795.
[13] (a) Bolaño, T.; Castarlenas, R.; Esteruelas, M. A.; Oñate, E. Organometallics 2007, 26, 2037.
(b) Bolaño, T.; Castarlenas, R.; Esteruelas, M. A.; Oñate, E. J. Am. Chem. Soc. 2007, 129, 8850.
(c) Buil, M. L.; Esteruelas, M. A.; Garcés, K.; Oñate, E. Organometallics 2009, 28, 5691.
[14] (a) Esteruelas, M. A.; González, A. I.; López, A. M.; Oñate, E. Organometallics 2003, 22, 414.
(b) Esteruelas, M. A.; González, A. I.; López, A. M.; Oñate, E. Organometallics 2004, 23, 4858.
(c) Castarlenas, R.; Esteruelas, M. A.; Oñate, E. Organometallics 2007, 26, 2129.
(d) Buil, M. L.; Esteruelas, M. A.; Garcés, K.; Oliván, M.; Oñate, E. Organometallics 2008, 27, 4680.
[15] Ozerov, O. V.; Watson, L. A.; Pink, M.; Caulton, K. G. J. Am. Chem. Soc. 2007, 129, 6003.
[16] Caulton, K. G. J. Organomet. Chem. 2001, 617~618, 56.
[17] (a) Ferrando, G.; Gérard, H.; Spivak, G. J.; Coalter Ⅲ, J. N.; Huffman, J. C.; Eisenstein, O.; Caulton, K. G. Inorg. Chem. 2001, 40, 6610.
(b) Ferrando-Miguel, G.; Gérard, H.; Eisenstein, O.; Caulton, K. G. Inorg. Chem. 2002, 41, 6440.
(c) Ferrando, G.; Coalter, J. N.; Gerard, H.; Huang, D.; Eisenstein, O.; Caulton, K. G. New J. Chem. 2003, 27, 1451.
(d) Grünwald, G.; Gevert, O.; Wolf, J.; González-Herrero, P.; Werner, H. Organometallics 1996, 15, 1960.
[18] (a) Espuelas, J.; Esteruelas, M. A.; Lahoz, F. J.; Oro, L. A.; Ruiz, N. J. Am. Chem. Soc. 1993, 115, 4683.
(b) Collado, A.; Esteruelas, M. A.; López, F.; Mascareñas, J. L.; Oñate, E.; Trillo, B. Organometallics 2010, 29, 4966.
(c) Collado, A.; Esteruelas, M. A.; Oñate, E. Organometallics 2011, 30, 1930.
[19] Spivak, G. J.; Coalter, J. N.; Olivan, M.; Eisenstein, O.; Caulton, K. G. Organometallics 1998, 17, 999.
[20] (a) Vougioukalakis, G. C.; Grubbs, R. H. Chem. Rev. 2010, 110, 1746.
(b) Grubbs, R. H. Tetrahedron 2004, 60, 7117.
(c) Trnka, T. M.; Grubbs, R. H. Acc. Chem. Res. 2001, 34, 18.
[21] Grubbs has reported the reaction of OsCl2(PPh3)3 with diphenylcyclopropene to afford OsCl2(=CHCH=CPh2)(PPh3)2, but no experimental evidence has been given, see:
(a) Nguyen, S. T.; Johnson, L. K.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1992, 114, 3974.
(b) Grubbs, R. H.; Schwab, P.; Nguyen, S. T. WO 9706185, 1997[Chem. Abstr. 1997, 126, 238816].
[22] Wilhelm, T. E.; Belderrain, T. R.; Brown, S. N.; Grubbs, R. H. Organometallics 1997, 16, 3867.
[23] (a) Volland, M. A. O.; Rominger, F.; Eisenträger, F.; Hofmann, P. J. Organomet. Chem. 2002, 641, 220.
Related syntheses of a series of ruthenium alkenylcarbenes RuCl2(=CHCH=CR1R2)(dtbpm) with a chelating ligand bis(di-tert-butyl-phosphanyl)methane t-Bu2PCH2Pt-Bu2(dtbpm) from the reactions of a dinuclear ruthenium hydride[RuH(μ2-Cl)-(dtbpm)]2 with propargylic chlorides HC≡CC(Cl)R1R2 have also been reported by Hofmann:
(b) Hansen, S. M.; Rominger, F.; Metz, M.; Hofmann, P. Chem.-Eur. J. 1999, 5, 557.
(c) Hansen, S. M.; Volland, M. A. O.; Rominger, F.; Eisenträger, F.; Hofmann, P. Angew. Chem., Int. Ed. 1999, 38, 1273.
[24] Amoroso, D.; Snelgrove, J. L.; Conrad, J. C.; Drouin, S. D.; Yap, G. P. A.; Fogg, D. E. Adv. Synth. Catal. 2002, 344, 757.
[25] Werner, H.; Jung, S.; Weberndörfer, B.; Wolf, J. Eur. J. Inorg. Chem. 1999, 1999, 951.
[26] Ferrando, G.; Caulton, K. G. Inorg. Chem. 1999, 38, 4168.
[27] (a) Bustelo, E.; Jiménez-Tenorio, M.; Mereiter, K.; Puerta, M. C.; Valerga, P. Organometallics 2002, 21, 1903.
(b) Esteruelas, M. A.; Oliván, M.; Oñate, E. Organometallics 1999, 18, 2953.
(c) Crochet, P.; Esteruelas, M. A.; López, A. M.; Martinez, M. P.; Oliván, M.; Oñate, E.; Ruiz, N. Organometallics 1998, 17, 4500.
[28] (a) Bruce, M. I. Chem. Rev. 1991, 91, 197.
(b) Bruce, M. I. Chem. Rev. 1998, 98, 2797.
[29] (a) Wen, T. B.; Yang, S. Y.; Zhou, Z. Y.; Lin, Z.; Lau, C. P.; Jia, G. Organometallics 2000, 19, 3757.
(b) Wen, T. B.; Zhou, Z. Y.; Jia, G. Angew. Chem., Int. Ed. 2001, 40, 1951.
(c) Wen, T. B.; Hung, W. Y.; Sung, H. H. Y.; Williams, I. D.; Jia, G. J. Am. Chem. Soc. 2005, 127, 2856.
(d) Wen, T. B.; Lee, K.-H.; Chen, J.; Hung, W. Y.; Bai, W.; Li, H.; Sung, H. H. Y.; Williams, I. D.; Lin, Z.; Jia, G. Organometallics 2016, 35, 1514.
[30] Collado, A.; Esteruelas, M. A.; López, F.; Mascareñas, J. L.; Oñate, E.; Trillo, B. Organometallics 2010, 29, 4966.
[31] Spivak, G. J.; Caulton, K. G. Organometallics 1998, 17, 5260.
[32] Hoffmann, P. R.; Caulton, K. G. J. Am. Chem. Soc. 1975, 97, 4221.
[33] Kondoh, A.; Yorimitsu, H.; Oshima, K. J. Am. Chem. Soc. 2007, 129, 4099. |