有机化学 ›› 2021, Vol. 41 ›› Issue (5): 2001-2007.DOI: 10.6023/cjoc202010036 上一篇 下一篇
研究论文
彭福涛a, 黄立梁a, 黄军海b,*( ), 冯煌迪a,*(
), 冯煌迪a,*( )
)
收稿日期:2020-10-28
修回日期:2020-12-30
发布日期:2021-02-07
通讯作者:
黄军海, 冯煌迪
基金资助:
Futao Penga, Liliang Huanga, Junhai Huangb,*( ), Huangdi Fenga,*(
), Huangdi Fenga,*( )
)
Received:2020-10-28
Revised:2020-12-30
Published:2021-02-07
Contact:
Junhai Huang, Huangdi Feng
About author:Supported by:文章分享
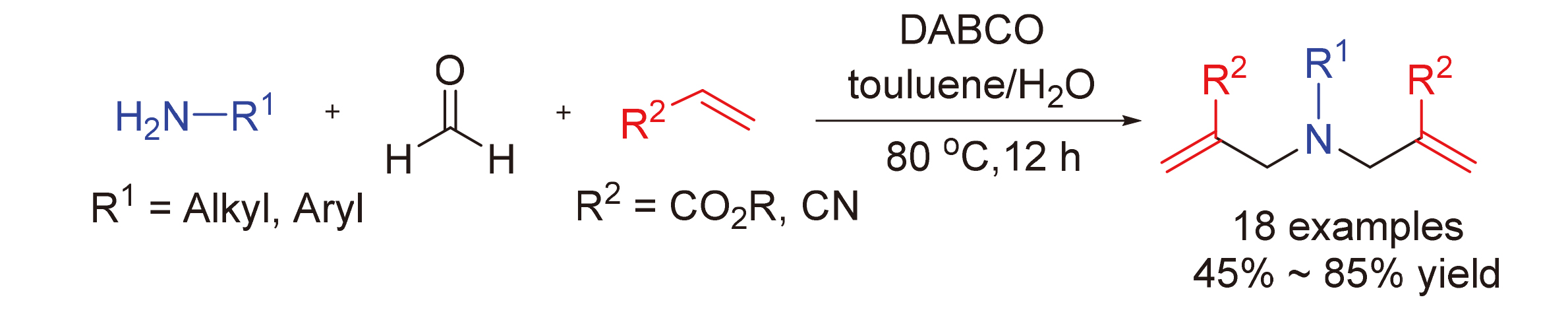
aza-Morita-Baylis-Hillman反应是一类非常重要的构建C—C键的人名反应, 被广泛应用于合成化学和药物化学领域. 报道了一类新颖的1,4-二氮杂二环[2.2.2]辛烷(DABCO)介导的二次aza-Morita-Baylis-Hillman串联反应. 该反应利用伯胺与甲醛能原位生成亚胺正离子的特征, 在甲苯与水的混合溶剂中, 实现了DABCO诱导的缺电子烯烃与亚胺正离子间的二次Mannich反应, 最终以中等到良好的产率获得了一系列氨基衍生的1,6-二烯化合物. 实验结果显示该三组分反应体系适用于一系列的苄胺、烷基胺和芳基胺底物, 有效避免了传统aza-Morita-Baylis-Hillman反应对底物胺的束缚, 为多样性1,6-二烯类化合物的合成提供了更加简洁的方法.
彭福涛, 黄立梁, 黄军海, 冯煌迪. aza-Morita-Baylis-Hillman反应二次串联构建氨基衍生的1,6-二烯化合物[J]. 有机化学, 2021, 41(5): 2001-2007.
Futao Peng, Liliang Huang, Junhai Huang, Huangdi Feng. Double aza-Morita-Baylis-Hillman Domino Reaction to Access Amino Derived 1,6-Dienes[J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2001-2007.
| Entry | Ratio of 1a/2a/3a | Additive | Solvent (V:V) | Yieldb/% |
|---|---|---|---|---|
| 1 | 0.5:2.0:3.0 | DABCO | Toluene | 45 |
| 2 | 0.5:2.0:3.0 | DBU | Toluene | 18 |
| 3 | 0.5:2.0:3.0 | DIEPA | Toluene | 12 |
| 4 | 0.5:2.0:3.0 | DMEDA | Toluene | 11 |
| 5 | 0.5:2.0:3.0 | DMAP | Toluene | 15 |
| 6 | 0.5:2.0:3.0 | PPh3 | Toluene | 20 |
| 7 | 0.5:2.0:3.0 | P(C5H11)3 | Toluene | 5 |
| 8 | 0.5:2.0:3.0 | DABCO | DMSO | 19 |
| 9 | 0.5:2.0:3.0 | DABCO | Dioxane | 9 |
| 10 | 0.5:2.0:3.0 | DABCO | THF | 40 |
| 11 | 0.5:2.0:3.0 | DABCO | EtOH | 30 |
| 12 | 0.5:2.0:3.0 | DABCO | CH3CN | 18 |
| 13 | 0.5:1.0:3.0 | DABCO | Toluene | 6 |
| 14 | 0.5:1.5:3.0 | DABCO | Toluene | 31 |
| 15 | 0.5:2.5:3.0 | DABCO | Toluene | 67 |
| 16 | 0.5:2.5:2.5 | DABCO | Toluene | 52 |
| 17 | 0.5:2.5:3.5 | DABCO | Toluene | 40 |
| 18c | 0.5:2.5:3.0 | DABCO | Toluene | 81 |
| 19 | 0.5:2.5:3.0 | DABCO | Toluene/H2O (24:1) | 80 |
| 20 | 0.5:2.5:3.0 | DABCO | Toluene/H2O (16:1) | 85 |
| 21 | 0.5:2.5:3.0 | DABCO | Toluene/H2O (8:1) | 65 |
| 22d | 0.5:2.5:3.0 | DABCO | Toluene | 0 |
| Entry | Ratio of 1a/2a/3a | Additive | Solvent (V:V) | Yieldb/% |
|---|---|---|---|---|
| 1 | 0.5:2.0:3.0 | DABCO | Toluene | 45 |
| 2 | 0.5:2.0:3.0 | DBU | Toluene | 18 |
| 3 | 0.5:2.0:3.0 | DIEPA | Toluene | 12 |
| 4 | 0.5:2.0:3.0 | DMEDA | Toluene | 11 |
| 5 | 0.5:2.0:3.0 | DMAP | Toluene | 15 |
| 6 | 0.5:2.0:3.0 | PPh3 | Toluene | 20 |
| 7 | 0.5:2.0:3.0 | P(C5H11)3 | Toluene | 5 |
| 8 | 0.5:2.0:3.0 | DABCO | DMSO | 19 |
| 9 | 0.5:2.0:3.0 | DABCO | Dioxane | 9 |
| 10 | 0.5:2.0:3.0 | DABCO | THF | 40 |
| 11 | 0.5:2.0:3.0 | DABCO | EtOH | 30 |
| 12 | 0.5:2.0:3.0 | DABCO | CH3CN | 18 |
| 13 | 0.5:1.0:3.0 | DABCO | Toluene | 6 |
| 14 | 0.5:1.5:3.0 | DABCO | Toluene | 31 |
| 15 | 0.5:2.5:3.0 | DABCO | Toluene | 67 |
| 16 | 0.5:2.5:2.5 | DABCO | Toluene | 52 |
| 17 | 0.5:2.5:3.5 | DABCO | Toluene | 40 |
| 18c | 0.5:2.5:3.0 | DABCO | Toluene | 81 |
| 19 | 0.5:2.5:3.0 | DABCO | Toluene/H2O (24:1) | 80 |
| 20 | 0.5:2.5:3.0 | DABCO | Toluene/H2O (16:1) | 85 |
| 21 | 0.5:2.5:3.0 | DABCO | Toluene/H2O (8:1) | 65 |
| 22d | 0.5:2.5:3.0 | DABCO | Toluene | 0 |
| [1] |
For selected reviews, see: (a) Basavaiah, D.; Jaganmohan Rao, A.; Satyanarayana, T. Chem. Rev. 2003, 103, 811.
pmid: 12630854 |
|
(b) Declerck, V.; Martinez, J.; Lamaty, F. Chem. Rev. 2009, 109, 1.
doi: 10.1021/cr068057c pmid: 12630854 |
|
|
(c) Xie, P. Z.; Huang, Y. Org. Biomol. Chem. 2015, 13, 8578.
doi: 10.1039/C5OB00865D pmid: 12630854 |
|
|
(d) Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659.
doi: 10.1021/cr300192h pmid: 12630854 |
|
|
(e) Cui, P. M.; Wang, C.; Ma, J. J.; Zhang, Y. Q.; Gao, Y. J.; Chang, X. H.; Zhang, D. N.; Zhou, H.; Zhang, H. Y. Chin. J. Org. Chem. 2005, 25, 763. (in Chinese).
pmid: 12630854 |
|
|
(崔朋雷, 王春, 马晶军, 张英群, 高勇军, 臧晓欢, 张冬暖, 周欣, 张红燕, 有机化学, 2005, 25, 763.)
pmid: 12630854 |
|
| [2] |
(a) Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447.
doi: 10.1021/cr900291g |
|
(b) Zhou, L. J.; Yuan, C. H.; Zeng, Y.; Wang, Q. J.; Wang, C.; Liu, M.; Wang, W.; Wu, Y. J.; Zheng, B.; Guo, H. C. Org. Lett. 2019, 21, 4882.
doi: 10.1021/acs.orglett.9b01783 |
|
|
(c) Peng, C.; Joy, A. Macromolecules 2014, 47, 125.
|
|
|
(d) Wu, M. Y.; Han, M. B.; Li, K. Z.; Wu, J.; Ding, K. L.; Lu, Y. X. J. Am. Chem. Soc. 2019, 141, 16362.
doi: 10.1021/jacs.9b07418 |
|
| [3] |
(a) Krafft, M. E.; Haxell, T. F. N. J. Am. Chem. Soc. 2005, 127, 10168.
pmid: 16028918 |
|
(b) Li, Y. Q.; Wang, H. J.; Huang, Z. Z. J. Org. Chem. 2016, 81, 4429.
doi: 10.1021/acs.joc.6b00684 pmid: 16028918 |
|
|
(c) Zhao, L. M.; Liu, K.; Li, D. F. J. Org. Chem. 2019, 84, 4429.
doi: 10.1021/acs.joc.8b03045 pmid: 16028918 |
|
|
(d) Li, Y. Q.; Huang, Z. Z. Acta Chim. Sinica 2017, 75, 280. (in Chinese).
doi: 10.6023/A16110587 pmid: 16028918 |
|
|
(李娅琼, 黄志真, 化学学报, 2017, 75, 280.)
doi: 10.6023/A16110587 pmid: 16028918 |
|
|
(e) Mi, X. L.; Luo, S. Z.; Cheng, J. P. J. Org. Chem. 2005, 70, 2338.
doi: 10.1021/jo048391d pmid: 16028918 |
|
|
(f) Yi, F. P.; Zhang, X.; Sun, H. Y.; Chen, S. H. Acta Chim. Sinica 2012, 70, 741. (in Chinese).
doi: 10.6023/A1110105 pmid: 16028918 |
|
|
(易封萍, 张旋, 孙海洋, 陈世洪, 化学学报, 2012, 70, 741.)
doi: 10.6023/A1110105 pmid: 16028918 |
|
| [4] |
(a) Bertenshaw, S.; Kahn, M. Tetrahedron Lett. 1989, 30, 2731.
doi: 10.1016/S0040-4039(00)99110-X |
|
(b) Ye, S. Q.; Wu, J. Tetrahedron Lett. 2009, 50, 6273.
doi: 10.1016/j.tetlet.2009.09.005 |
|
|
(c) Zhang, X.; Zhou, Z.; Xu, H. Y.; Xu, X. F.; Yu, X. Y.; Yi, W. Org. Lett. 2019, 21, 7248.
doi: 10.1021/acs.orglett.9b02462 |
|
| [5] |
(a) Chen, J.; Li, J. J.; Wang, J. Z.; Li, H.; Wang, W.; Guo, Y. W. Org. Lett. 2015, 17, 2214.
doi: 10.1021/acs.orglett.5b00811 pmid: 12201741 |
|
(b) Walton, M. C.; Yang, Y. F.; Hong, X.; Houk, K. N.; Overman, L. E. Org. Lett. 2015, 17, 6166.
doi: 10.1021/acs.orglett.5b03171 pmid: 12201741 |
|
|
(c) Jiang, B.; Xiao, B. X.; Ouyang, Q.; Liang, H. P.; Du, W.; Chen, Y. C. Org. Lett. 2019, 21, 3310.
doi: 10.1021/acs.orglett.9b01058 pmid: 12201741 |
|
|
(d) Patil, S. N.; Liu, F. J. Org. Chem. 2008, 73, 4476.
doi: 10.1021/jo702762u pmid: 12201741 |
|
|
(e) Shi, F.; Luo, S. W.; Tao, Z. L.; He, L.; Yu, J.; Tu, S. J.; Gong, L. Z. Org. Lett. 2011, 13, 4680.
doi: 10.1021/ol201898x pmid: 12201741 |
|
|
(f) Mergott, D. J.; Frank, S. A.; Roush, W. R. Org. Lett. 2002, 4, 3157.
pmid: 12201741 |
|
| [6] |
Shi Shi. M.; Xu Y. M. J. Org. Chem. 2003, 68, 4784.
doi: 10.1021/jo034114f |
| [7] |
(a) Zhang, L.; Liu, H. L.; Qiao, G. Y.; Hou, Z. F.; Liu, Y.; Xiao, Y. M.; Guo, H. C. J. Am. Chem. Soc. 2015, 137, 4316.
doi: 10.1021/jacs.5b01138 |
|
(b) Qi, J. F.; Zheng, J.; Cui, S. L. Org. Lett. 2018, 20, 1355.
doi: 10.1021/acs.orglett.8b00108 |
|
| [8] |
(a) Feng, H. D.; Jia, H. H.; Sun, Z. H. J. Org. Chem. 2014, 79, 11812.
doi: 10.1021/jo502349a |
|
(b) Feng, H. D.; Jia, H. H.; Sun, Z. H. Adv. Synth. Catal. 2015, 357, 2447.
doi: 10.1002/adsc.v357.11 |
|
|
(c) Zhang, Y. Z.; Huang, L. L.; Li, X. Y.; Wang, L.; Feng, H. D. J. Org. Chem. 2019, 84, 5046.
doi: 10.1021/acs.joc.8b03244 |
|
|
(d) Li, H. Q.; Feng, H. D.; Wang, F.; Huang, L. L. J. Org. Chem. 2019, 84, 10380.
doi: 10.1021/acs.joc.9b01547 |
|
|
(e) Feng, H. D.; Zhang, Y. Z.; Zhang, Z. D.; Chen, F. B.; Huang, L. L. Eur. J. Org. Chem. 2019,1931.
|
|
|
(f) Zhang, Y. Z.; Feng, H. D.; Liu, X. H.; Huang, L. L. Eur. J. Org. Chem. 2018,2039.
|
|
|
(g) Xu, X. J.; Van de Eycken, E. V.; Feng, H. D. Chin. J. Chem. 2020, 38, 1780.
doi: 10.1002/cjoc.v38.12 |
|
| [9] |
(a) Xu, M. Y.; Jiang, W. T.; Li, Y.; Xu, Q. H.; Zhou, Q. L.; Yang, S.; Xiao, B. J. Am. Chem. Soc. 2019, 141, 7582.
doi: 10.1021/jacs.9b02776 |
|
(b) Döbbelin, M.; Azcune, I.; Bedu, M.; Ruiz de Luzuriaga, A.; Genua, A.; Jovanovski, V.; Cabañero, G.; Odriozola, I. Chem. Mater. 2012, 24, 1583.
doi: 10.1021/cm203790z |
|
|
(c) Yan, T. B.; Liu, Y. H.; Shen, Y. H. Chin. J. Org. Chem. 2018, 38, 2491. (in Chinese).
doi: 10.6023/cjoc201805001 |
|
|
(颜廷斌, 刘跃辉, 沈悦海, 有机化学, 2018, 38, 2491.)
doi: 10.6023/cjoc201805001 |
|
|
(d) Huang, L.; Cai, Y.; Zhang, H.-J.; Zheng, C.; Dai, L.-X.; You, S.-L. CCS Chem. 2019, 1, 106.
|
|
|
(e) Zhu, M.; Zheng, C.; Zhang, X.; You, S.-L. J. Am. Chem. Soc. 2019, 141, 2636.
doi: 10.1021/jacs.8b12965 |
|
| [10] |
(a) Eren, T. N.; Graff, B.; Lalevee, J.; Avci, D. Prog. Org. Coat. 2019, 128, 156.
|
|
(b) Gao, S. F.; Zhang, Y. C.; Li, J. M.; Zhang, B. Yang, Y.; Hu, M. Q. Chin. J. Org. Chem. 2019, 39, 1953. (in Chinese).
doi: 10.6023/cjoc201812037 |
|
|
(高粟繁, 张艳春, 李家明, 张斌, 杨雨, 胡孟奇, 有机化学, 2019, 39, 1953.)
doi: 10.6023/cjoc201812037 |
|
|
(c) Zhao, Y. J.; Cao, Y. K.; Chend, H. Z.; Zhuang, F.; Wu, C.; Yoon, G.; Zhu, W. W.; Su, Y.; Zheng, S. Q.; Liu, Z. G.; Cheon, S. H. Bioorg. Med. Chem. 2019, 27, 963.
|
|
| [11] |
(a) Guo, Y. H.; Wang, G. D.; Wei, L.; Wan, J. P. J. Org. Chem. 2019, 84, 2984.
doi: 10.1021/acs.joc.8b02897 |
|
(b) Zheng, X. X.; Wan, J. P. Adv. Synth. Catal. 2019, 361, 5690.
doi: 10.1002/adsc.201901054 |
|
|
(c) Fu, L. Q.; Cao, X. J.; Wan, J. P.; Liu, Y. Y. Chin. J. Chem. 2020, 38, 254.
doi: 10.1002/cjoc.v38.3 |
|
|
(d) Chen, X. P.; Ma, Z. W.; Wang, C. C.; Liu, J. T.; Wu, J. S. Chin. J. Org. Chem. 2019, 39, 3176. (in Chinese).
doi: 10.6023/cjoc201905017 |
|
|
(陈晓培, 马志伟, 王川川, 刘俊桃, 吴金松, 有机化学, 2019, 39, 3176.)
doi: 10.6023/cjoc201905017 |
|
| [12] |
(a) Yu, C. Z.; Liu, B.; Hu, L. Q. J. Org. Chem. 2001, 66, 5413.
pmid: 11485463 |
|
(b) Cai, J. X.; Zhou, Z. H.; Zhao, G. F.; Tang, C. C. Org. Lett. 2002, 4, 4723.
doi: 10.1021/ol027197f pmid: 11485463 |
|
|
(c) Li, Y. X.; Liu, L.; Kong, D. L.; Wang, D.; Feng, W. C.; Yue, T.; Li, C. J. J. Org. Chem. 2015, 80, 6283.
doi: 10.1021/acs.joc.5b00728 pmid: 11485463 |
|
| [13] |
(a) Wang, J. Y.; Shen, Q. Y.; Li, P. Z.; Peng, Y. Q.; Song, G. H. Org. Biomol. Chem. 2014, 12, 5597.
doi: 10.1039/C4OB01055H |
|
(b) Zhao, P. F.; Feng, H. D.; Pan, H. R.; Sun, Z. H.; Tong, M. C. Org. Chem. Front. 2017, 4, 37.
doi: 10.1039/C6QO00499G |
|
|
(c) Liu, B. Y.; Xu, X. J.; Huang, L. L.; Feng, H. D. Chin. J. Org. Chem. 2020, 40, 1290. (in Chinese).
doi: 10.6023/cjoc201911020 |
|
|
(刘博瑜, 徐仙君, 黄立梁, 冯煌迪, 有机化学, 2020, 40, 1290.)
doi: 10.6023/cjoc201911020 |
|
| [14] |
Pandey, A. K.; Han, S. H.; Mishra, N. K.; Kang, D.; Lee, S. H.; Chun, R.; Hong, S.; Park, J. S.; Kim, I. S. ACS Catal. 2018, 8, 742.
doi: 10.1021/acscatal.7b03812 |
| [15] |
Shinohara, I.; Okue, M.; Yamada, Y.; Nagaoka, H. Tetrahedron Lett. 2003, 44, 4649.
doi: 10.1016/S0040-4039(03)01093-1 |
| [1] | 陈乡萍, 孟晨湘, 李梦娜, 楚尚敏, 朱欣欣, 许凯, 刘澜涛, 王涛, 张凤华, 李飞. 水相中抗坏血酸钠促进铁催化合成含硫芳香伯胺化合物[J]. 有机化学, 2023, 43(8): 2800-2807. |
| [2] | 曾成富, 何媛, 李清, 董琳. Ir(III)催化新型三组分串联三氟乙氧基化反应并一锅法构建复杂酰胺化合物[J]. 有机化学, 2023, 43(3): 1115-1123. |
| [3] | 李硕, 王明亮, 周来运, 王兰芝. 磁性纳米负载对甲苯磺酸催化串联合成稠合多环的1,5-苯并氧氮杂䓬类化合物[J]. 有机化学, 2023, 43(11): 3977-3988. |
| [4] | 石云, 肖婷, 夏冬, 杨文超. 三氟甲硫基自由基引发不饱和烃的串联反应[J]. 有机化学, 2022, 42(9): 2715-2727. |
| [5] | 赵晓正, 凌琴琴, 曹桂妍, 火星, 赵小龙, 苏瀛鹏. 炔丙醇类化合物参与的环化反应研究进展[J]. 有机化学, 2022, 42(9): 2605-2639. |
| [6] | 代增进, 张绪穆, 殷勤. 铵盐为胺源的不对称还原胺化反应研究进展[J]. 有机化学, 2022, 42(8): 2261-2274. |
| [7] | 周旭煜, 张爱君, 张庆庆, 刘庆安, 宣俊. 可见光诱导4-色满酮合成: 醋酸碘苯促进的α-酮酸与邻-烯丙氧基芳醛的自由基串联环化反应[J]. 有机化学, 2022, 42(8): 2488-2495. |
| [8] | 侯金松, 杨高升. 三(邻二甲胺基苄基)钇催化脂肪胺对烯腈的插入串联反应[J]. 有机化学, 2022, 42(7): 2070-2078. |
| [9] | 袁飞, 赵艳, 郭青松, 尹福丹, 赖金荣, 念倍芳, 张明, 汤峨. 乙烯基硒盐参与的串联反应合成1-[1-(胺基)环丙基]酮化合物[J]. 有机化学, 2022, 42(6): 1759-1769. |
| [10] | 孙鑫, 屈超凡, 马超蕊, 赵筱薇, 柴国璧, 江智勇. 光氧化还原催化串联自由基加成反应构建1,4-二酮官能团化喹喔啉-2(1H)-酮衍生物[J]. 有机化学, 2022, 42(5): 1396-1406. |
| [11] | 肖立伟, 刘光仙, 任萍, 吴彤桐, 卢玉伟, 孔洁. 单质硫: 合成含硫杂环的优质硫源[J]. 有机化学, 2022, 42(4): 1002-1012. |
| [12] | 乔辉杰, 杨利婷, 陈雅, 王嘉琳, 孙武轩, 董昊博, 王云威. 温和条件下高效合成咪唑并杂环-肼类衍生物的三组分串联反应[J]. 有机化学, 2022, 42(4): 1188-1197. |
| [13] | 罗享豪, 谢益碧, 黄年玉, 王龙. 基于原位捕获异腈的Ugi四组分反应及其后修饰串联反应: 一锅法合成含氮杂环化合物[J]. 有机化学, 2022, 42(3): 838-846. |
| [14] | 洪科苗, 黄晶晶, 姚铭瀚, 徐新芳. 氮宾/炔烃复分解串联反应研究进展[J]. 有机化学, 2022, 42(2): 344-352. |
| [15] | 王明亮, 尹刘燕, 温甜甜, 张晓, 高杰, 王兰芝. 多官能团化的1,5-苯并二氮杂䓬类化合物的绿色合成[J]. 有机化学, 2022, 42(1): 160-171. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||