有机化学 ›› 2021, Vol. 41 ›› Issue (8): 3223-3234.DOI: 10.6023/cjoc202103049 上一篇 下一篇
所属专题: 有机电合成虚拟专辑
研究论文
高君青a, 翁信军b, 马聪b, 徐学涛a,*( ), 方萍b,*(
), 方萍b,*( ), 梅天胜b,*(
), 梅天胜b,*( )
)
收稿日期:2021-03-26
修回日期:2021-04-26
发布日期:2021-05-14
通讯作者:
徐学涛, 方萍, 梅天胜
作者简介:基金资助:
Junqing Gaoa, Xinjun Wengb, Cong Mab, Xuetao Xua( ), Ping Fangb(
), Ping Fangb( ), Tiansheng Meib(
), Tiansheng Meib( )
)
Received:2021-03-26
Revised:2021-04-26
Published:2021-05-14
Contact:
Xuetao Xu, Ping Fang, Tiansheng Mei
About author:Supported by:文章分享
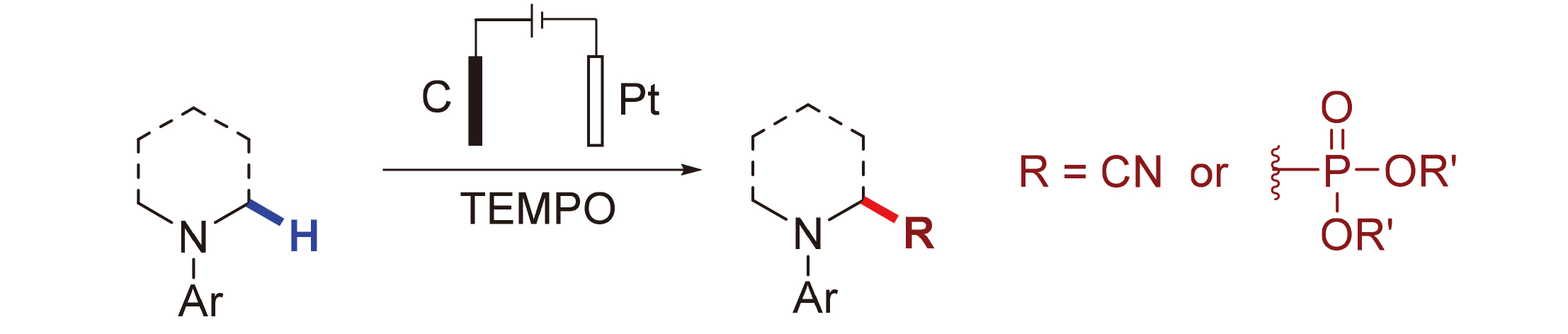
探究了无金属电化学氧化氰化和膦酸酯基化反应, 其中2,2,6,6-四甲基哌啶-氮-氧化物(TEMPO)降低了底物的电极电位, 避免了某些富电子芳香胺在电化学条件下的过氧化反应. 该方法具有良好的官能团兼容性, 是在温和条件下合成α-氨基腈和α-氨基膦酸酯的一种有效且实用的方法, 通过研究表明产物通过Shono氧化形成亚胺物种来实现的.
高君青, 翁信军, 马聪, 徐学涛, 方萍, 梅天胜. 无金属参与的电化学促进2,2,6,6-四甲基哌啶-氮-氧化物(TEMPO)介导的环胺α-氰化和膦酰化反应[J]. 有机化学, 2021, 41(8): 3223-3234.
Junqing Gao, Xinjun Weng, Cong Ma, Xuetao Xu, Ping Fang, Tiansheng Mei. Electrochemical 2,2,6,6-tetramethylpiperidinyl-N-oxyl (TEMPO)-Mediated α-Cyanation and Phosphonylation of Cyclic Amines with Metal-Free Conditions[J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3223-3234.
| Entry | Variation from standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 99 (97)c |
| 2 | 4-CN-TEMPO instead of TEMPO | 71 |
| 3 | 4-COOH-TEMPO instead of TEMPO | 48 |
| 4 | ABNO instead of TEMPO | 67 |
| 5 | PhCO2ABNO instead of TEMPO | 42 |
| 6 | Keto-ABNO instead of TEMPO | 33 |
| 7 | TBAOAc instead of TBAPF6 | 46 |
| 8 | TBAClO4 instead of TBAPF6 | 53 |
| 9 | NaClO4 instead of TBAPF6 | 68 |
| 10 | H2O instead of CF3CH2OH | 69 |
| 11 | HFIP instead of CF3CH2OH | 91 |
| 12 | MeOH instead of CF3CH2OH | 78 |
| 13 | Isopropanol instead of CF3CH2OH | 38 |
| 14 | No current | N.R. |
| 15 | No TEMPO, no CH3CH2OH | 29 |
| 16 | No TEMPO | 45 |
| Entry | Variation from standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 99 (97)c |
| 2 | 4-CN-TEMPO instead of TEMPO | 71 |
| 3 | 4-COOH-TEMPO instead of TEMPO | 48 |
| 4 | ABNO instead of TEMPO | 67 |
| 5 | PhCO2ABNO instead of TEMPO | 42 |
| 6 | Keto-ABNO instead of TEMPO | 33 |
| 7 | TBAOAc instead of TBAPF6 | 46 |
| 8 | TBAClO4 instead of TBAPF6 | 53 |
| 9 | NaClO4 instead of TBAPF6 | 68 |
| 10 | H2O instead of CF3CH2OH | 69 |
| 11 | HFIP instead of CF3CH2OH | 91 |
| 12 | MeOH instead of CF3CH2OH | 78 |
| 13 | Isopropanol instead of CF3CH2OH | 38 |
| 14 | No current | N.R. |
| 15 | No TEMPO, no CH3CH2OH | 29 |
| 16 | No TEMPO | 45 |


| [1] |
(a) Weinstock, L. M.; Davis, P.; Handelsman, B.; Tull, R. J. J. Org. Chem. 1967, 32, 2823.
doi: 10.1021/jo01284a040 pmid: 4745833 |
|
(b) Matier, W. L.; Owens, D. A.; Comer, W. T.; Deitchman, D.; Ferguson, H. C. Seidehamel, R. J.; Young, J. R. J. Med. Chem. 1973, 16, 901.
pmid: 4745833 |
|
|
(c) Enders, D.; Shilvock, J. P. Chem. Soc. Rev. 2000, 29, 359.
doi: 10.1039/a908290e pmid: 4745833 |
|
| [2] |
(a) Shono, T.; Matsumura, Y.; Uchida, K.; Tsubata, K.; Makino, A. J. Org. Chem. 1984, 49, 300.
doi: 10.1021/jo00176a016 pmid: 25147957 |
|
(b) Myers, E. L.; de Vries, J. G.; Aggarwal, V. K. Angew. Chem. Int. Ed. 2007, 46, 1893.
doi: 10.1002/(ISSN)1521-3773 pmid: 25147957 |
|
|
(c) Kabeshov, M. A.; Musio, B.; Murray, P. R.; Browne, D. L.; Ley, S. V. Org. Lett. 2014, 16, 4618.
doi: 10.1021/ol502201d pmid: 25147957 |
|
| [3] |
(a) Moeller, K. D.; Wong, P. L. Bioorg. Med. Chem. Lett. 1992, 2, 739.
doi: 10.1016/S0960-894X(00)80403-5 |
|
(b) Frankowski, K. J.; Liu, R.; Milligan, G. L.; Moeller, K. D.; Aube, J. Angew. Chem. Int. Ed. 2015, 54, 10555.
doi: 10.1002/anie.201504775 |
|
| [4] |
Myers, E. L.; de Vries, J. G.; Aggarwal, V. K. Angew. Chem. 2007, 119, 1925.
doi: 10.1002/(ISSN)1521-3757 |
| [5] |
(a) Rappoport, Z. The Chemistry of the Cyano Group, Interscience Publishers, London, 1970.
|
|
(b) Larock, R. C. Comprehensive Organic Transformations: A Guide to Functional Group Preparations, VCH, New York, 1989.
|
|
| [6] |
For selected examples on Fe-catalyzed, see:
|
|
(a) Singhal, S.; Jain, S. L.; Sain, B. Adv. Synth. Catal. 2010, 352, 1338.
doi: 10.1002/adsc.v352:8 |
|
|
(b) Liu, P.; Liu, Y.; Wong, E. L-M.; Xiang, S.; Che, C.-M. Chem. Sci. 2011, 2, 2187.
doi: 10.1039/c1sc00234a |
|
|
(c) Sun, C. L.; Li, B. J.; Shi, Z. J. Chem. Rev. 2011, 111, 1293.
doi: 10.1021/cr100198w |
|
|
(d) Wagner, A.; Han, W.; Mayer, P.; Ofial, A. R. Adv. Synth. Catal. 2013, 355, 3058.
doi: 10.1002/adsc.v355.14/15 |
|
|
(e) Patil, M.; Kapdi, A. R.; Kumar, A. V. RSC Adv. 2015, 5, 54505.
doi: 10.1039/C5RA10552H |
|
|
(f) Wagner, A.; Hampel, N.; Zipse, H.; Ofial, A. R. Org. Lett. 2015, 17, 4770.
doi: 10.1021/acs.orglett.5b02319 |
|
|
(g) Nauth, A. M.; Otto, N.; Opatz, T. Adv. Synth. Catal. 2015, 357, 3424.
doi: 10.1002/adsc.201500698 |
|
|
(h) Panwar, V.; Kumar, P.; Bansal, A.; Ray, S. S.; Jain, S. L. Appl. Catal., 2015, 498, 25.
doi: 10.1016/j.apcata.2015.03.018 |
|
|
(i) Zhang, L.; Gu, X.; Lu, P.; Wang, Y. Tetrahedron 2016, 72, 2359.
doi: 10.1016/j.tet.2016.03.061 |
|
|
(j) Huang, M.; Deng, Q.; Gao, Q.; Shi, J.; Zhang, X.; Xiong, Y. Asian J. Org. Chem. 2018, 7, 404.
doi: 10.1002/ajoc.v7.2 |
|
| [7] |
For selected examples on Cu-catalyzed, see:
pmid: 27934338 |
|
(a) Li, Z.; Li, C.-J. Eur. J. Org. Chem., 2005, 2005, 3173.
doi: 10.1002/(ISSN)1099-0690 pmid: 27934338 |
|
|
(b) Han, W.; Ofial, A. R. Chem. Commun. 2009, 5024.
pmid: 27934338 |
|
|
(c) Ogibin, Y. N.; Elinson, M. N.; Nikishin, G. I. Russ. Chem. Rev. 2009, 78, 89.
doi: 10.1070/RC2009v078n02ABEH003886 pmid: 27934338 |
|
|
(d) Boess, E.; Schmitz, C.; Klussmann, M. J. Am. Chem. Soc. 2012, 134, 5317.
doi: 10.1021/ja211697s pmid: 27934338 |
|
|
(e) Zhang, G.; Ma, Y.; Cheng, G.; Liu, D.; Wang, R. Org. Lett. 2014, 16, 656.
doi: 10.1021/ol500045p pmid: 27934338 |
|
|
(f) Fandrick, D. R.; Hart, C. A.; Okafor, I. S.; Mercadante, M. A.; Sanyal, S.; Masters, J. T.; Sarvestani, M.; Fandrick, K. R.; Stockdill, J. L.; Grinberg, N.; Gonnella, N.; Lee, H.; Senanayake, C. H. Org. Lett. 2016, 18, 6192.
pmid: 27934338 |
|
|
(g) Liu, Y.; Wang, C.; Xue, D.; Xiao, M.; Li, C.; Xiao, J. Chem.- Eur. J. 2017, 23, 3051.
doi: 10.1002/chem.201604749 pmid: 27934338 |
|
| [8] |
For selected examples on Ir-catalyzed, see: a Rueping,
pmid: 30160970 |
|
(a) Rueping,
doi: 10.1039/c1cc15643h pmid: 30160970 |
|
|
(b) Verma, D.; Verma, S.; Sinha, A. K.; Jain, S. L. ChemPlusChem 2013, 78, 860.
doi: 10.1002/cplu.201300196 pmid: 30160970 |
|
|
(c) Ide, T.; Shimizu, K.; Egami, H.; Hamashima, Y. Tetrahedron Lett. 2018, 59, 3258.
doi: 10.1016/j.tetlet.2018.07.030 pmid: 30160970 |
|
|
(d) Yilmaz, O.; Oderinde, M. S.; Emmert, M. H. J. Org. Chem. 2018, 83, 11089.
doi: 10.1021/acs.joc.8b01700 pmid: 30160970 |
|
| [9] |
For selected examples on Ru-catalyzed, see:
|
|
(a) Murahashi, S. I.; Komiya, N.; Terai, H.; Nakae, T. J. Am. Chem. Soc. 2003, 125, 15312.
doi: 10.1021/ja0390303 |
|
|
(b) North, M. Angew. Chem. Int. Ed. 2004, 43, 4126.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(c) Murahashi, S.; Komiya, N.; Terai, H. Angew. Chem. Int. Ed. 2005, 44, 6931.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(d) Murahashi, S. I.; Nakae, T.; Terai, H.; Komiya, N. J. Am. Chem. Soc. 2008, 130, 11005.
doi: 10.1021/ja8017362 |
|
|
(e) Verma, S.; Jain, S. L.; Sain, B. ChemCatChem 2011, 3, 1329.
doi: 10.1002/cctc.201100111 |
|
|
(f) Verma, S.; Jain, S. L.; Sain, B. Catal. Lett. 2011, 141, 882.
doi: 10.1007/s10562-011-0582-6 |
|
|
(g) Schümperli, M. T.; Hammond, C.; Hermans, I. ACS Catal. 2012, 2, 1108.
doi: 10.1021/cs300212q |
|
|
(h) Panwar, V.; Ray, S. S.; Jain, S. L. Tetrahedron Lett. 2015, 56, 4184.
doi: 10.1016/j.tetlet.2015.05.033 |
|
| [10] |
For selected examples on Ti-catalyzed, see:
|
|
(a) Rueping, M.; Zoller, J.; Fabry, D. C.; Poscharny, K.; Koenigs, R. M.; Weirich, T. E.; Mayer, J. Chem.-Eur. J. 2012, 18, 3478.
doi: 10.1002/chem.201103242 |
|
|
(b) Kumar, P.; Varma, S.; Jain, S. L. J. Mater. Chem. A 2014, 2, 4514.
|
|
|
(c) Nauth, A. M.; Schechtel, E.; Doren, R.; Tremel, W.; Opatz, T. J. Am. Chem. Soc. 2018, 140, 14169.
doi: 10.1021/jacs.8b07539 |
|
| [11] |
For selected examples on other metal-catalyzed, see:
pmid: 30998010 |
|
(a) Singhal, S.; Jain, S. L.; Sain, B. Chem. Commun. 2009, 2371.
pmid: 30998010 |
|
|
(b) Zhang, Y.; Peng, H.; Zhang, M.; Cheng, Y.; Zhu, C. Chem. Commun. 2011, 47, 2354.
doi: 10.1039/C0CC03844J pmid: 30998010 |
|
|
(c) Sakai, N.; Mutsuro, A.; Ikeda, R.; Konakahara, T. Synlett 2013, 24, 1283.
doi: 10.1055/s-00000083 pmid: 30998010 |
|
|
(d) Lin, A.; Peng, H.; Abdukader, A.; Zhu, C. Eur. J. Org. Chem. 2013, 2013, 7286.
doi: 10.1002/ejoc.v2013.32 pmid: 30998010 |
|
|
(e) Yang, W.; Wei, L.; Yi, F.; Cai, M. Tetrahedron 2016, 72, 4059.
doi: 10.1016/j.tet.2016.05.037 pmid: 30998010 |
|
|
(f) Ping, Y.; Ding, Q.; Peng, Y. ACS Catal. 2016, 6, 5989.
doi: 10.1021/acscatal.6b01632 pmid: 30998010 |
|
|
(g) Liang, W.; Zhang, T.; Liu, Y.; Huang, Y.; Liu, Z.; Liu, Y.; Yang, B.; Zhou, X.; Zhang, J. ChemSusChem 2018, 11, 3586.
doi: 10.1002/cssc.v11.20 pmid: 30998010 |
|
|
(h) Patil, M. R.; Dedhia, N. P.; Kapdi, A. R.; Kumar, A. V. J. Org. Chem. 2018, 83, 4477.
doi: 10.1021/acs.joc.8b00203 pmid: 30998010 |
|
|
(i) Casado-Sanchez, A.; Uygur, M.; Gonzalez-Munoz, D.; Aguilar-Galindo, F.; Nova-Fernandez, J. L.; Arranz-Plaza, J.; Diaz-Tendero, S.; Cabrera, S.; Mancheno, O. G.; Aleman, J. J. Org. Chem. 2019, 84, 6437.
doi: 10.1021/acs.joc.9b00520 pmid: 30998010 |
|
| [12] |
For selected examples on non-metal catalyzed, such as: AIBN, TBAI, TBHP, TBP, see:
|
|
(a) Liu, L. H.; Wang, Z. K.; Fu, X. F.; Yan, C. H. Org. Lett. 2012, 14, 5692.
doi: 10.1021/ol302708r |
|
|
(b) Zhang, C.; Liu, C.; Shao, Y.; Bao, X.; Wan, X. Chem.-Eur. J. 2013, 19, 17917.
doi: 10.1002/chem.201303296 |
|
|
(c) Wang, H.; Shao, Y.; Zheng, H.; Wang, H.; Cheng, J.; Wan, X. Chem.-Eur. J. 2015, 21, 18333.
doi: 10.1002/chem.201502733 |
|
|
(d) Zhang, Z.; Gu, K.; Bao, Z.; Xing, H.; Yang, Q.; Ren, Q. Tetrahedron 2017, 73, 3118.
doi: 10.1016/j.tet.2017.04.033 |
|
|
(e) Liu, P. Y.; Zhang, C.; Zhao, S. C.; Yu, F.; Li, F.; He, Y. P. J. Org. Chem. 2017, 82, 12786.
doi: 10.1021/acs.joc.7b02021 |
|
|
(f) Sun, M. X.; Wang, Y. F.; Xu, B. H.; Ma, X. Q.; Zhang, S. J. Org. Biomol. Chem. 2018, 16, 19717.
|
|
|
(g) Ullah, B.; Zhou, Y.; Chen, J.; Bao, Z.; Yang, Y.; Yang, Q.; Ren, Q.; Zhang, Z. Tetrahedron Lett. 2019, 60, 348.
doi: 10.1016/j.tetlet.2018.12.050 |
|
| [13] |
For selected examples on non-metal catalyzed, such as: PhIOAc2 and AcOH, see:
|
|
(a) Shu, X. Z.; Xia, X. F.; Yang, Y. F.; Ji, K. G.; Liu, X. Y.; Liang, Y. M. J. Org. Chem. 2009, 74, 7464.
doi: 10.1021/jo901583r |
|
|
(b) Ueda, H.; Yoshida, K.; Tokuyama, H. Org. Lett. 2014, 16, 4194.
doi: 10.1021/ol5018883 |
|
| [14] |
For selected examples on other non-metal catalyzed, see:
pmid: 25147957 |
|
(a) Rueping, M.; Zhu, S.; Koenigs, R. M. Chem. Commun. 2011, 47, 8679.
doi: 10.1039/c1cc12907d pmid: 25147957 |
|
|
(b) Allen, J. M.; Lambert, T. H. J. Am. Chem. Soc. 2011, 133, 1260.
doi: 10.1021/ja109617y pmid: 25147957 |
|
|
(c) Ushakov, D. B.; Gilmore, K.; Kopetzki, D.; McQuade, D. T.; Seeberger, P. H. Angew. Chem. Int. Ed. 2014, 53, 557.
pmid: 25147957 |
|
|
(d) Kabeshov, M. A.; Musio, B.; Murray, P. R.; Browne, D. L.; Ley, S. V. Org. Lett. 2014, 16, 4618.
doi: 10.1021/ol502201d pmid: 25147957 |
|
|
(e) Shen, H.; Zhang, X.; Liu, Q.; Pan, J.; Hu, W.; Xiong, Y.; Zhu, X. Tetrahedron Lett. 2015, 56, 5628.
doi: 10.1016/j.tetlet.2015.08.058 pmid: 25147957 |
|
|
(f) Orejarena Pacheco, J.C.; Lipp,, A.; Nauth,, A. M.; Acke,, F.; Dietz,, J. P.; Opatz,, T. Chem.-Eur. J. 2016, 22, 5409.
doi: 10.1002/chem.201504845 pmid: 25147957 |
|
|
(g) Fu, N.; Li, L.; Yang, Q.; Luo, S. Org. Lett. 2017, 19, 2122.
doi: 10.1021/acs.orglett.7b00746 pmid: 25147957 |
|
|
(h) Chen, W.; Ma, L.; Paul, A.; Seidel, D. Nat. Chem. 2018, 10, 165.
doi: 10.1038/nchem.2871 pmid: 25147957 |
|
|
(i) Periasamy, M.; Rao, G. Tetrahedron 2018, 74, 7209.
doi: 10.1016/j.tet.2018.10.058 pmid: 25147957 |
|
| [15] |
For selected examples on other mediated catalyzed, see:
pmid: 29707945 |
|
(a) Wagner, A.; Ofial, A. R. J. Org. Chem. 2015, 80, 2848.
doi: 10.1021/jo502846c pmid: 29707945 |
|
|
(b) Wang, F.; Rafiee, M.; Stahl, S. S. Angew. Chem. Int. Ed. 2018, 57, 6686.
doi: 10.1002/anie.v57.22 pmid: 29707945 |
|
|
(c) Lennox, A. J. J.; Goes, S. L.; Webster, M. P.; Koolman, H. F.; Djuric, S. W.; Stahl, S. S. J. Am. Chem. Soc. 2018, 140, 11227.
doi: 10.1021/jacs.8b08145 pmid: 29707945 |
|
|
(d) Nutting, J. E.; Rafiee, M.; Stahl, S. S. Chem. Rev. 2018, 118, 4834.
doi: 10.1021/acs.chemrev.7b00763 pmid: 29707945 |
|
| [16] |
(a) To, W. P.; Tong, G. S.; Lu, W.; Ma, C.; Liu, J.; Chow, A. L.; Che, C. M. Angew. Chem. Int. Ed. 2012, 51, 2654.
doi: 10.1002/anie.201108080 |
|
(b) Ushakov, D. B.; Gilmore, K.; Kopetzki, D.; McQuade, D. T.; Seeberger, P. H. Angew. Chem. Int. Ed. 2014, 53, 557.
|
|
|
(c) Orejarena Pacheco, J.C.; Lipp,, A.; Nauth,, A. M.; Acke,, F.; Dietz,, J. P.; Opatz,, T. Chem.-Eur. J. 2016, 22, 5409.
doi: 10.1002/chem.201504845 |
|
|
(d) Nauth, A. M.; Lipp, A.; Lipp, B.; Opatz, T. Eur. J. Org. Chem. 2017, 2099.
|
|
|
(e) Nauth, A. M.; Orejarena Pacheco, J. C.; Pusch, S.; Opatz, T. Eur. J. Org. Chem. 2017, 6966.
|
|
|
(f) Nauth, A. M.; Schechtel, E.; Doren, R.; Tremel, W.; Opatz, T. J. Am. Chem. Soc. 2018, 140, 14169.
doi: 10.1021/jacs.8b07539 |
|
|
(g) Yi, B.; Yan, N.; Yi, N.; Xie, Y.; Wen, X.; Au, C.-T.; Lan, D. Chem.-Asian J. 2020, 15, 4302.
|
|
| [17] |
For selected reviews on electrochemical C—H functionalization, see:
|
|
(a) Yang, Q.-L.; Fang, P.; Mei, T.-S. Chin. J. Chem. 2018, 36, 338.
doi: 10.1002/cjoc.v36.4 |
|
|
(b) Ma, C.; Fang, P.; Mei, T.-S. ACS. Catal. 2018, 8, 7179.
|
|
|
(c) Yang, F.; Zhang, H.; Liu, X.; Wang, B.; Ackermann, L. Chin. J. Org. Chem. 2019, 39, 59.
doi: 10.6023/cjoc201808017 |
|
|
(d) Meng, Z.-Y.; Feng, C.-T.; Xu, K. Chin. J. Org. Chem. 2021, 41, 2535. (in Chinese)
doi: 10.6023/cjoc202012013 |
|
|
(蒙泽银, 冯承涛, 徐坤, 有机化学, 2021, 41, 2535.)
doi: 10.6023/cjoc202012013 |
|
| [18] |
For selected reviews or highlights on electrochemistry, see:
|
|
(a) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230.
doi: 10.1021/acs.chemrev.7b00397 |
|
|
(b) Tang, S.; Liu, Y.; Lei, A. Chem 2018, 4, 27.
doi: 10.1016/j.chempr.2017.10.001 |
|
|
(c) Ma, H. X.; Mei, T. S. Chin. J. Org. Chem. 2020, 40, 3982. (in Chinese)
doi: 10.6023/cjoc202000079 |
|
|
(马红星, 梅天胜, 有机化学, 2020, 40, 3982.)
doi: 10.6023/cjoc202000079 |
|
|
(d) Jiang, Y.-Y.; Zeng, C.-C. Chin. J. Org. Chem. 2020, 40, 2999. (in Chinese)
doi: 10.6023/cjoc202000057 |
|
|
(蒋洋叶, 曾程初, 有机化学, 2020, 40, 2999.)
doi: 10.6023/cjoc202000057 |
|
|
(e) Ye, Z. H.; Zhang, F. Z. Chin. J. Org. Chem. 2020, 40, 241. (in Chinese)
doi: 10.6023/cjoc202000002 |
|
|
(叶增辉, 张逢质, 有机化学, 2020, 40, 241.)
doi: 10.6023/cjoc202000002 |
|
|
(f) Wang, X. Y.; Xu, X. T.; Wang, Z. H.; Fang, P.; Mei, T. S. Chin. J. Org. Chem. 2020, 40, 3738. (in Chinese)
doi: 10.6023/cjoc202003022 |
|
|
(王向阳, 徐学涛, 王振华, 方萍, 梅天胜, 有机化学, 2020, 40, 3738.)
doi: 10.6023/cjoc202003022 |
|
| [19] |
(a) Shono, T.; Matsumura, Y.; Tsubata, K. J. Am. Chem. Soc. 1981, 103, 1172.
doi: 10.1021/ja00395a029 |
|
(b) Shono, T.; Matsumura, Y.; Uchida, K.; Tsubata, K.; Makino, A. J. Org. Chem. 1984, 49, 300.
doi: 10.1021/jo00176a016 |
|
| [20] |
Gao, P. S.; Weng, X. J.; Wang, Z. H.; Zheng, C.; Sun, B.; Chen, Z. H.; You, S. L.; Mei, T. S. Angew. Chem. Int. Ed. 2020, 59, 15254.
doi: 10.1002/anie.v59.35 |
| [21] |
(a) Steckhan, V. E. Angew. Chem. 1986, 98, 681.
doi: 10.1002/(ISSN)1521-3757 pmid: 24500279 |
|
(b) Ogibin, Y. N.; Elinson, M. N.; Nikishin, G. I. Russ. Chem. Rev. 2009, 78, 89.
doi: 10.1070/RC2009v078n02ABEH003886 pmid: 24500279 |
|
|
(c) Francke, R.; Little, R. D. Chem. Soc. Rev. 2014, 43, 2492.
doi: 10.1039/c3cs60464k pmid: 24500279 |
|
| [22] |
(a) Quint, V.; Chouchène, N.; Askri, M.; Lalevée, J.; Gaumont, A.-C.; Lakhdar, S. Org. Chem. Front. 2019, 6, 41.
doi: 10.1039/C8QO00985F pmid: 31525939 |
|
(b) Huang, M.; Dai, J.; Cheng, X.; Ding, M. Org. Lett. 2019, 21, 7759.
doi: 10.1021/acs.orglett.9b02707 pmid: 31525939 |
|
| [23] |
Rao, G. A.; Periasamy, M. Synthesis 2018, 50, 617.
doi: 10.1055/s-0036-1590943 |
| [24] |
Yi, B.; Yan, N.; Yi, N.; Xie, Y.; Wen, X.; Au, C.-T.; Lan, D. RSC Adv. 2019, 9, 29721.
doi: 10.1039/C9RA06120G |
| [25] |
Wang, H. L.; Li, X. C.; Wu, F.; Wan, B. S. Tetrahedron Lett. 2012, 53, 681.
|
| [26] |
Lin, B. Z.; Shi, S. S.; Lin, R. C.; Cui, Y. Q.; Fang, M. J.; Tang, G.; Zhao, Y. F. J. Org. Chem. 2018, 83, 6754.
doi: 10.1021/acs.joc.8b00674 |
| [27] |
Beke, D.; Bárczai, B. M.; Fcze, L. Chem. Ber. 1962, 95, 1054.
doi: 10.1002/(ISSN)1099-0682 |
| [1] | 徐利军, 李宗军, 韩福社, 高翔. N,N-二甲基甲酰胺促进的富勒烯稠合噁唑啉衍生物的合成[J]. 有机化学, 2024, 44(1): 242-250. |
| [2] | 蔡远林, 吕亚, 聂桂花, 金智超, 池永贵. 氮杂环卡宾催化合成氰基化合物的研究进展[J]. 有机化学, 2023, 43(9): 3135-3145. |
| [3] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [4] | 钟赟哲, 陈颖, 俞磊, 周宏伟. 电化学介导羧酸与醇的酯化反应[J]. 有机化学, 2023, 43(8): 2855-2863. |
| [5] | 张周, 郭钰, 羊静, 吴丹, 王佳昕, 洪欣玥, 蔡佩君, 荣良策. 电化学促进咪唑并[1,2-a]吡啶与二氯(溴)乙烷及碘仿的卤化反应[J]. 有机化学, 2023, 43(6): 2104-2109. |
| [6] | 张俊颖, 赵晓静, 李干鹏, 何永辉. 室温下电化学合成保护型有机硼酸RB(dan)[J]. 有机化学, 2023, 43(5): 1815-1823. |
| [7] | 杜琳琳, 张华. 芳烃与烷烃化合物参与的光化学与电化学硼化反应[J]. 有机化学, 2023, 43(5): 1726-1741. |
| [8] | 潘永周, 蒙秀金, 王迎春, 何慕雪. 电化学固定CO2构建羧酸衍生物的研究进展[J]. 有机化学, 2023, 43(4): 1416-1434. |
| [9] | 孙丽, 宋国欣, 韩家乐, 李继玉, 赵月, 杨璐华, 张峰, 赵坤, 毛比明. Morita-Baylis-Hillman加合物和N-羟基邻苯二甲酰亚胺的电化学烯丙基烷基化形成C(sp3)—C(sp3)键[J]. 有机化学, 2023, 43(4): 1574-1583. |
| [10] | 黄嘉为, 李潇漫, 徐亮, 韦玉. α-酮酸与硫酚的电化学脱羧偶联: 一种合成硫代酸酯的新方法[J]. 有机化学, 2023, 43(2): 756-762. |
| [11] | 魏琬絜, 詹磊, 高雷, 黄国保, 马献力. 电化学合成C-磺酰基化合物的研究进展[J]. 有机化学, 2023, 43(1): 17-35. |
| [12] | 危斌, 周子龙, 秦景灏, 严泽宇, 郭嘉程, 雷澍, 谢叶香, 欧阳旋慧, 宋仁杰. 氧杂蒽与亚磺酸钠的电化学氧化C(sp3)—H磺酰化反应[J]. 有机化学, 2023, 43(1): 186-194. |
| [13] | 王川川, 马志伟, 侯学会, 杨龙华, 陈亚静. N-Ts氰胺在有机合成中的研究与应用[J]. 有机化学, 2023, 43(1): 74-93. |
| [14] | 李海琼, 尹梦云, 谢芬芬, 张正兵, 韩盼, 敬林海. 通过电化学Appel反应合成腈[J]. 有机化学, 2022, 42(7): 2229-2235. |
| [15] | 顾清云, 程振凤, 曾小宝. 电化学氧化三氟甲基亚磺酸钠与α-羰基二硫缩烯酮的三氟甲基化反应[J]. 有机化学, 2022, 42(5): 1537-1544. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||