有机化学 ›› 2021, Vol. 41 ›› Issue (11): 4391-4399.DOI: 10.6023/cjoc202106010 上一篇 下一篇
所属专题: 热点论文虚拟合集
研究论文
收稿日期:2021-06-05
修回日期:2021-07-16
发布日期:2021-08-17
通讯作者:
郭海明
基金资助:
Chao Xiaa, Dongchao Wangb, Haiming Guoa,b( )
)
Received:2021-06-05
Revised:2021-07-16
Published:2021-08-17
Contact:
Haiming Guo
Supported by:文章分享
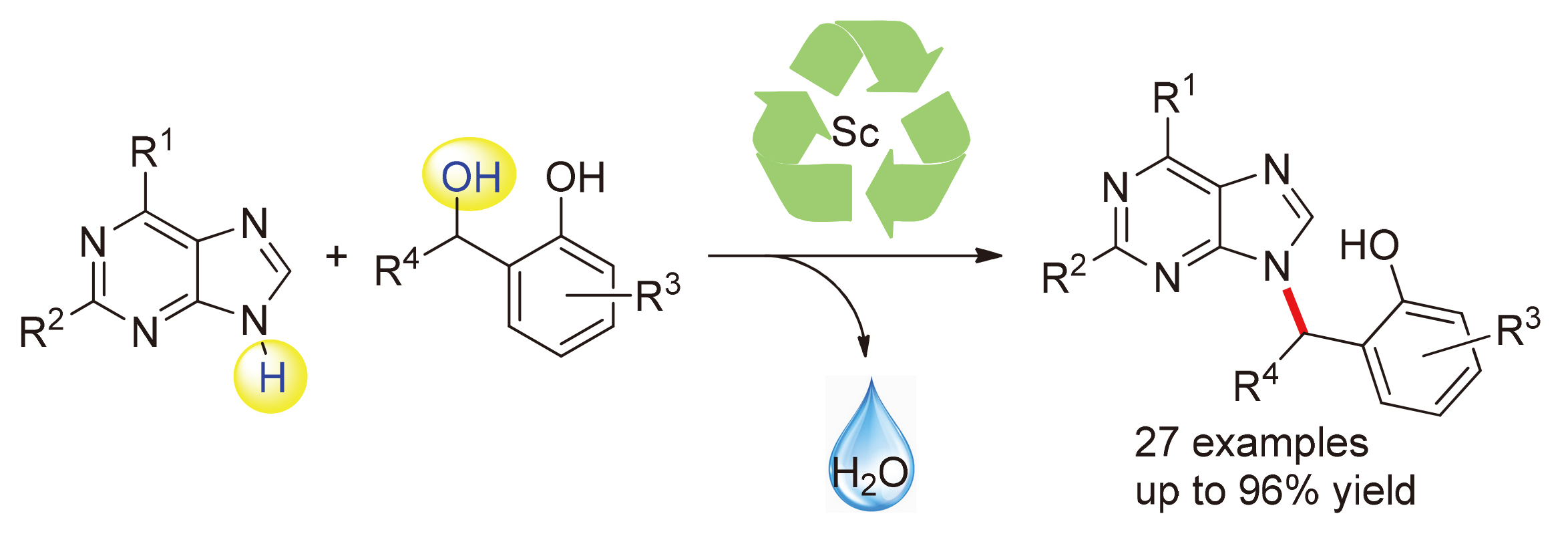
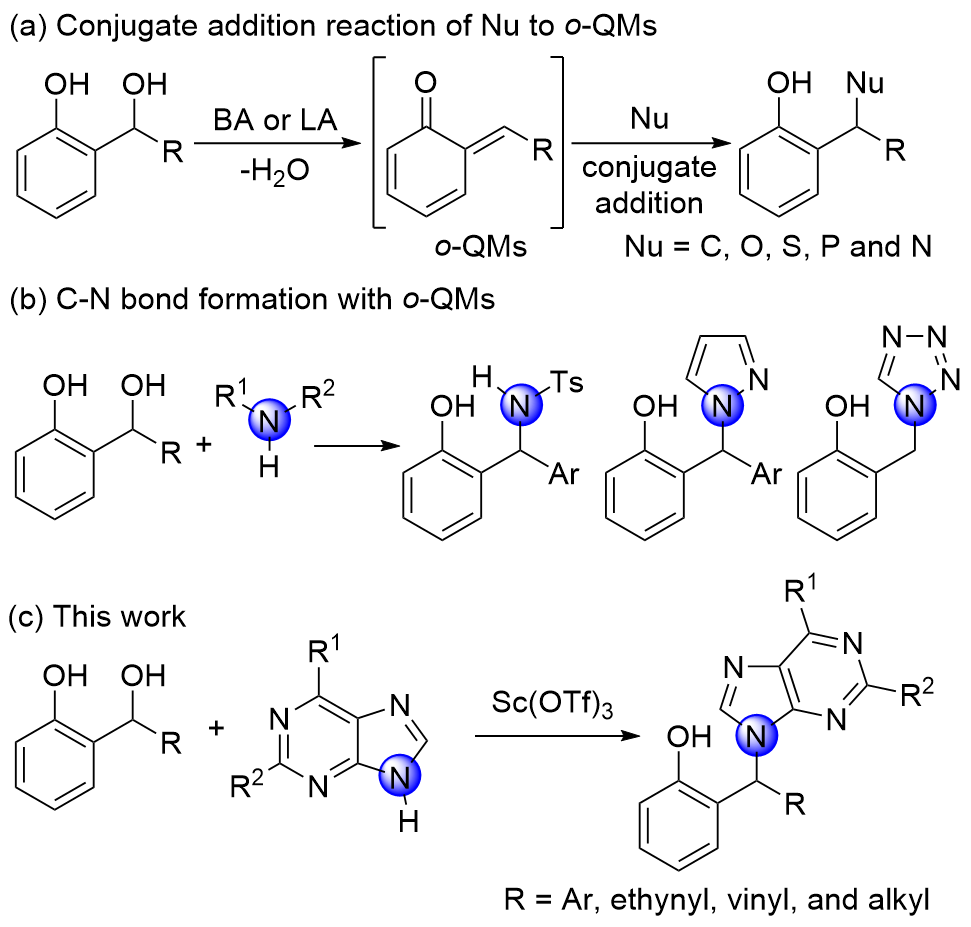
通过三氟甲磺酸钪催化, 在温和条件下实现了邻羟基苄醇与嘌呤的烷基化反应. 该C—N键形成的过程可能是通过邻亚甲基苯醌中间体进行的. 以优秀的产率(最高可达96%)高效地合成了一系列非环核苷类似物, 底物范围广. 反应规模放大后, 产率保持不变.
夏超, 王东超, 郭海明. 三氟甲磺酸钪催化嘌呤与邻羟基苄醇反应构建非环核苷[J]. 有机化学, 2021, 41(11): 4391-4399.
Chao Xia, Dongchao Wang, Haiming Guo. Sc(OTf)3-Catalyzed Reaction of Purines with o-Hydroxybenzyl Alcohols for Construction of Acyclic Nucleosides[J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4391-4399.
| Entry | LA (x) | Solvent | t/h | Yieldb/% |
|---|---|---|---|---|
| 1 | In(OTf)3 (20) | DCM | 12 | 75 |
| 2 | Cu(OTf)2 (20) | DCM | 12 | 68 |
| 3 | Fe(OTf)3 (20) | DCM | 12 | 71 |
| 4 | Sc(OTf)3 (20) | DCM | 12 | 95 |
| 5 | Y(OTf)3 (20) | DCM | 12 | 18 |
| 6 | Mg(OTf)2 (20) | DCM | 12 | 10 |
| 7 | Zn(OTf)2 (20) | DCM | 12 | NR |
| 8 | TfOH (10) | DCM | 12 | 20 |
| 9 | Sc(OTf)3 (10) | DCM | 5 | 94 |
| 10 | Sc(OTf)3 (5) | DCM | 10 | 94 |
| 11 | Sc(OTf)3 (2.5) | DCM | 24 | 68 |
| 12c | Sc(OTf)3 (2.5) | DCM | 24 | 58 |
| 13d | Sc(OTf)3 (2.5) | DCM | 24 | 61 |
| 14e | Sc(OTf)3 (2.5) | DCM | 24 | 55 |
| 15 | Sc(OTf)3 (5) | Toluene | 10 | 74 |
| 16 | Sc(OTf)3 (5) | Et2O | 10 | NR |
| 17 | Sc(OTf)3 (5) | Acetone | 10 | 81 |
| 18 | Sc(OTf)3 (5) | THF | 10 | NR |
| 19 | Sc(OTf)3 (5) | EA | 10 | 78 |
| Entry | LA (x) | Solvent | t/h | Yieldb/% |
|---|---|---|---|---|
| 1 | In(OTf)3 (20) | DCM | 12 | 75 |
| 2 | Cu(OTf)2 (20) | DCM | 12 | 68 |
| 3 | Fe(OTf)3 (20) | DCM | 12 | 71 |
| 4 | Sc(OTf)3 (20) | DCM | 12 | 95 |
| 5 | Y(OTf)3 (20) | DCM | 12 | 18 |
| 6 | Mg(OTf)2 (20) | DCM | 12 | 10 |
| 7 | Zn(OTf)2 (20) | DCM | 12 | NR |
| 8 | TfOH (10) | DCM | 12 | 20 |
| 9 | Sc(OTf)3 (10) | DCM | 5 | 94 |
| 10 | Sc(OTf)3 (5) | DCM | 10 | 94 |
| 11 | Sc(OTf)3 (2.5) | DCM | 24 | 68 |
| 12c | Sc(OTf)3 (2.5) | DCM | 24 | 58 |
| 13d | Sc(OTf)3 (2.5) | DCM | 24 | 61 |
| 14e | Sc(OTf)3 (2.5) | DCM | 24 | 55 |
| 15 | Sc(OTf)3 (5) | Toluene | 10 | 74 |
| 16 | Sc(OTf)3 (5) | Et2O | 10 | NR |
| 17 | Sc(OTf)3 (5) | Acetone | 10 | 81 |
| 18 | Sc(OTf)3 (5) | THF | 10 | NR |
| 19 | Sc(OTf)3 (5) | EA | 10 | 78 |
| [1] |
(a) Willis, N. J.; Bray, C. D. Chem.-Eur. J. 2012, 18, 9160.
doi: 10.1002/chem.201200619 pmid: 27513764 |
|
(b) Bai, W.-J.; David, J. G.; Feng, Z.-G.; Weaver, M. G.; Wu, K.-L.; Pettus, T. R. R. Acc. Chem. Res. 2014, 47, 3655.
doi: 10.1021/ar500330x pmid: 27513764 |
|
|
(c) Jaworski, A. A.; Scheidt, K. A. J. Org. Chem. 2016, 81, 10145.
pmid: 27513764 |
|
|
(d) Yang, B.; Gao, S. Chem. Soc. Rev. 2018, 47, 7926.
doi: 10.1039/C8CS00274F pmid: 27513764 |
|
| [2] |
(a) Meisinger, N.; Roiser, L.; Monkowius, U.; Himmelsbach, M.; Robiette, R.; Waser, M. Chem.-Eur. J. 2017, 23, 5137.
doi: 10.1002/chem.201700171 pmid: 30407018 |
|
(b) Suneja, A.; Schneider, C. Org. Lett. 2018, 20, 7576.
doi: 10.1021/acs.orglett.8b03311 pmid: 30407018 |
|
|
(c) Pandit, R. P.; Kim, S. T.; Ryu, D. H. Angew. Chem., Int. Ed. 2019, 58, 13427.
doi: 10.1002/anie.v58.38 pmid: 30407018 |
|
| [3] |
(a) Bu, H.-Z.; Li, H.-H.; Luo, W.-F.; Luo, C.; Qian, P.-C.; Ye, L.-W. Org. Lett. 2020, 22, 648.
doi: 10.1021/acs.orglett.9b04421 |
|
(b) Feng, S.; Yang, B.; Chen, T.; Wang, R.; Deng, Y.-H.; Shao, Z. J. Org. Chem. 2020, 85, 5231.
doi: 10.1021/acs.joc.9b03302 |
|
|
(c) Huang, H.-M.; Wu, X.-Y.; Leng, B.-R.; Zhu, Y.-L.; Meng, X.-C.; Hong, Y.; Jiang, B.; Wang, D.-C. Org. Chem. Front. 2020, 7, 414.
doi: 10.1039/C9QO01343A |
|
|
(d) Shi, H.; Wang, L.; Li, S.-S.; Liu, Y.; Xu, L. Org. Chem. Front. 2020, 7, 747.
doi: 10.1039/D0QO00038H |
|
|
(e) Tanaka, K.; Asada, Y.; Hoshino, Y.; Honda, K. Org. Biomol. Chem. 2020, 18, 8074.
doi: 10.1039/D0OB01151G |
|
|
(f) Tu, M.-S.; Liu, S.-J.; Zhong, C.; Zhang, S.; Zhang, H.; Zheng, Y.-L.; Shi, F. J. Org. Chem. 2020, 85, 5403.
doi: 10.1021/acs.joc.0c00119 |
|
|
(g) You, Y.; Li, T.-T.; Yuan, S.-P.; Xie, K.-X.; Wang, Z.-H.; Zhao, J.-Q.; Zhou, M.-Q.; Yuan, W.-C. Chem. Commun. 2020, 56, 439.
doi: 10.1039/C9CC08316B |
|
| [4] |
(a) Mei, G.-J.; Zhu, Z.-Q.; Zhao, J.-J.; Bian, C.-Y.; Chen, J.; Chen, R.-W.; Shi, F. Chem. Commun. 2017, 53, 2768.
doi: 10.1039/C6CC09775H |
|
(b) Lam, H.; Qureshi, Z.; Wegmann, M.; Lautens, M. Angew. Chem., Int. Ed. 2018, 57, 16185.
doi: 10.1002/anie.v57.49 |
|
|
(c) Sun, M.; Ma, C.; Zhou, S.-J.; Lou, S.-F.; Xiao, J.; Jiao, Y.; Shi, F. Angew. Chem., Int. Ed. 2019, 58, 8703.
doi: 10.1002/anie.v58.26 |
|
|
(d) Suneja, A.; Loui, H. J.; Schneider, C. Angew. Chem., Int. Ed. 2020, 59, 5536.
doi: 10.1002/anie.v59.14 |
|
|
(e) Yan, R.-J.; Liu, B.-X.; Xiao, B.-X.; Du, W.; Chen, Y.-C. Org. Lett. 2020, 22, 4240.
doi: 10.1021/acs.orglett.0c01283 |
|
|
(f) Zhou, S.-J.; Sun, M.; Wang, J.-Y.; Yu, X.-Y.; Lu, H.; Zhang, Y.-C.; Shi, F. Eur. J. Org. Chem. 2020, 4301.
|
|
| [5] |
Xia, C.; Wang, D.-C.; Qu, G.-R.; Guo, H.-M. Org. Chem. Front. 2020, 7, 1474.
doi: 10.1039/D0QO00128G |
| [6] |
(a) Wang, Y.; Zhang, C.; Wang, H.; Jiang, Y.; Du, X.; Xu, D. Adv. Synth. Catal. 2017, 359, 791.
doi: 10.1002/adsc.v359.5 pmid: 29420039 |
|
(b) Wu, J.-L.; Wang, J.-Y.; Wu, P.; Mei, G.-J.; Shi, F. Org. Chem. Front. 2017, 4, 2465.
doi: 10.1039/C7QO00649G pmid: 29420039 |
|
|
(c) Zhou, J.; Huang, W.-J.; Jiang, G.-F. Org. Lett. 2018, 20, 1158.
doi: 10.1021/acs.orglett.8b00025 pmid: 29420039 |
|
|
(d) Wu, Q.; Li, G.-L.; Yang, S.; Shi, X.-Q.; Huang, T.-Z.; Du, X.-H.; Chen, Y. Org. Biomol. Chem. 2019, 17, 3462.
doi: 10.1039/C9OB00283A pmid: 29420039 |
|
|
(e) Chu, M.-M.; Chen, X.-Y.; Wang, Y.-F.; Qi, S.-S.; Jiang, Z.-H.; Xu, D.-Q.; Xu, Z.-Y. J. Org. Chem. 2020, 85, 9491.
doi: 10.1021/acs.joc.9b03479 pmid: 29420039 |
|
|
(f) Zhang, Y.-Z.; Sheng, F.-T.; Zhu, Z.; Li, Z.-M.; Zhang, S.; Tan, W.; Shi, F. Org. Biomol. Chem. 2020, 18, 5688.
doi: 10.1039/D0OB01230K pmid: 29420039 |
|
| [7] |
Lai, Z.; Wang, Z.; Sun, J. Org. Lett. 2015, 17, 6058.
doi: 10.1021/acs.orglett.5b03072 |
| [8] |
(a) Guo, W.; Wu, B.; Zhou, X.; Chen, P.; Wang, X.; Zhou, Y.-G.; Liu, Y.; Li, C. Angew. Chem., Int. Ed. 2015, 54, 4522.
doi: 10.1002/anie.201409894 |
|
(b) Lai, Z.; Sun, J. Synlett 2016, 27, 555.
doi: 10.1055/s-00000083 |
|
|
(c) Roy, D.; Panda, G. Tetrahedron 2018, 74, 6270.
doi: 10.1016/j.tet.2018.09.009 |
|
| [9] |
(a) Huang, H.; Kang, J. Y. Org. Lett. 2017, 19, 5988.
doi: 10.1021/acs.orglett.7b03019 pmid: 29064709 |
|
(b) Gu, X.; Yuan, H.; Jiang, J.; Wu, Y.; Bai, W.-J. Org. Lett. 2018, 20, 7229.
doi: 10.1021/acs.orglett.8b03158 pmid: 29064709 |
|
| [10] |
(a) Chen, M.; Han, Y.; Ma, D.; Wang, Y.; Lai, Z.; Sun, J. Chin. J. Chem. 2018, 36, 587.
doi: 10.1002/cjoc.v36.7 |
|
(b) Pelayo, S. G.; López, L. A. Eur. J. Org. Chem. 2017, 2017, 6003.
doi: 10.1002/ejoc.201701183 |
|
|
(c) Chen, L.-M.; Zhao, J.; Xia, A.-J.; Guo, X.-Q.; Gan, Y.; Zhou, C.; Yang, Z.-J.; Yang, J.; Kang, T.-R. Org. Biomol. Chem. 2019, 17, 8561.
doi: 10.1039/C9OB01780A |
|
|
(d) Osyanin, V. A.; Osipov, D. V.; Nakushnov, V. Y.; Zemtsova, M. N.; Klimochkin, Y. N. Chem. Heterocycl. Compd. 2015, 51, 984.
doi: 10.1007/s10593-016-1808-8 |
|
| [11] |
(a) Kelley, J. L.; Kelsey, J. E.; Hall, W. R.; Krochmal, M. P.; Schaeffer, H. J. J. Med. Chem. 1981, 24, 753.
pmid: 30939009 |
|
(b) Vandenriessche, F.; Snoeck, R.; Janssen, G.; Hoogmartens, J.; Aerschot, A. V.; Clercq, E. D.; Herdewijn, P. J. Med. Chem. 1992, 35, 1458.
doi: 10.1021/jm00086a015 pmid: 30939009 |
|
|
(c) Sekiyama, T.; Hatsuya, S.; Tanaka, Y.; Uchiyama, M.; Ono, N.; Iwayama, S.; Oikawa, M.; Suzuki, K.; Okunishi, M.; Tsuji, T. J. Med. Chem. 1998, 41, 1284.
pmid: 30939009 |
|
|
(d) Hakimelahi, G. H.; Ly, T. W.; Movahedi, A. A. M.; Jain, M. L.; Zakerinia, M.; Davari, H.; Mei, H.-C.; Sambaiah, T.; Moshfegh, A. A.; Hakimelahi, S. J. Med. Chem. 2001, 44, 3710.
pmid: 30939009 |
|
|
(e) Hernández, A.-I.; Balzarini, J.; Karlsson, A.; Camarasa, M.-J.; Pérez, M.-J. J. Med. Chem. 2002, 45, 4254.
doi: 10.1021/jm011128+ pmid: 30939009 |
|
|
(f) Clercq, E. D. J. Med. Chem. 2019, 62, 7322.
doi: 10.1021/acs.jmedchem.9b00175 pmid: 30939009 |
|
| [12] |
(a) Palazzolo, M. A.; Nigro, M. J.; Iribarren, A. M.; Lewkowicz, E. S. Eur. J. Org. Chem. 2016, 2016, 921.
pmid: 28829913 |
|
(b) Derudas, M.; Vanpouille, C.; Carta, D.; Zicari, S.; Andrei, G.; Snoeck, R.; Brancale, A.; Margolis, L.; Balzarini, J.; McGuigan, C. J. Med. Chem. 2017, 60, 7876.
doi: 10.1021/acs.jmedchem.7b01009 pmid: 28829913 |
|
|
(c) Lee, S.-J.; Ahn, J.-G.; Seo, J.; Ha, H.-J.; Cho, C.-W. Org. Biomol. Chem. 2018, 16, 9477.
doi: 10.1039/C8OB02754D pmid: 28829913 |
|
|
(d) Zhang, H.; Xie, M.; Qu, G.; Chang, J. Org. Lett. 2019, 21, 120.
doi: 10.1021/acs.orglett.8b03555 pmid: 28829913 |
|
|
(e) Baddi, L.; Ouzebla, D.; Mansouri, A.-E.; Smietana, M.; Vasseur, J.-J.; Lazrek, H. B. Nucleosides, Nucleotides Nucleic Acids 2021, 40, 43.
doi: 10.1080/15257770.2020.1826516 pmid: 28829913 |
|
| [13] |
(a) Sun, H.-L.; Chen, F.; Xie, M.-S.; Guo, H.-M.; Qu, G.-R.; He, Y.-M.; Fan, Q.-H. Org. Lett. 2016, 18, 2260.
doi: 10.1021/acs.orglett.6b00869 pmid: 28901778 |
|
(b) Zhou, P.; Xie, M.-S.; Qu, G.-R.; Li, R.-L.; Guo, H.-M. Asian J. Org. Chem. 2016, 5, 1100.
doi: 10.1002/ajoc.201600251 pmid: 28901778 |
|
|
(c) Liang, L.; Xie, M.-S.; Qin, T.; Zhu, M.; Qu, G.-R.; Guo, H.-M. Org. Lett. 2017, 19, 5212.
doi: 10.1021/acs.orglett.7b02482 pmid: 28901778 |
|
|
(d) Wang, H.-X.; Yu, L.-L.; Xie, M.-S.; Wu, J.; Qu, G-R.; Ding, K.-L.; Guo, H.-M. Chem.-Eur. J. 2018, 24, 1425.
doi: 10.1002/chem.v24.6 pmid: 28901778 |
|
|
(e) Liang, T.; Xie, M.-S.; Qu, G.-R.; Guo, H.-M. Asian J. Org. Chem. 2019, 8, 1405.
doi: 10.1002/ajoc.201900227 pmid: 28901778 |
|
|
(f) Hao, E.-J.; Li, G.-X.; Liang, Y.-R.; Xie, M.-S.; Wang, D.-C.; Jiang, X.-H.; Cheng, J.-Y.; Shi, Z.-X.; Wang, Y.; Guo, H.-M. J. Med. Chem. 2021, 64, 2077.
doi: 10.1021/acs.jmedchem.0c01717 pmid: 28901778 |
|
|
(g) Xia, C.; Wang, D.-C.; Qu, G.-R.; Guo, H.-M. Org. Chem. Front. 2021, 8, 2569.
doi: 10.1039/D1QO00272D pmid: 28901778 |
| [1] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [2] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [3] | 陈天煜, 韩峰, 李双艳, 刘建平, 陈建辉, 徐清. 无过渡金属参与杂环甲基化合物与醇的选择性有氧碳-烷基化反应[J]. 有机化学, 2022, 42(9): 2914-2924. |
| [4] | 南光明, 詹靖波, 原春鸣, 文丽荣, 李明. 三乙胺促进的邻亚甲基苯醌与β-羰基硫代酰胺的[4+2]环加成反应构建功能化4H-色烯衍生物[J]. 有机化学, 2021, 41(9): 3682-3691. |
| [5] | 刘颖杰, 韩莹徽, 林立青, 许颖. 电化学催化下的多氟烷基化反应研究进展[J]. 有机化学, 2021, 41(3): 934-946. |
| [6] | 胡智宇, 姜国芳, 祝志强, 龚伯桢, 谢宗波, 乐长高. 深共融溶剂促进的亨利-傅克烷基化串联反应[J]. 有机化学, 2021, 41(1): 325-332. |
| [7] | 韩满意, 潘虹, 姚紫云, 李琦. 四丁基溴化铵催化的布鲁克重排/烷基化反应[J]. 有机化学, 2020, 40(12): 4274-4283. |
| [8] | 吴敦奇, 成轩, 刘炎开, 成果, 管笑宇, 邓清海. 新型手性钳形PNN类配体在钯催化不对称烯丙基烷基化反应中的应用[J]. 有机化学, 2020, 40(10): 3362-3370. |
| [9] | 张硕, 赵宁, 李庆刚, 张嘉祺, 侯梓桐, 刘一帆, 于一涛, 彭丹, 王峰, 李冰, 李金辉. Sc(Ⅲ)催化胺对邻亚甲基苯醌氮杂迈克尔加成反应合成贝蒂碱衍生物[J]. 有机化学, 2019, 39(3): 709-719. |
| [10] | 张硕, 彭丹, 赵宁, 于一涛, 王峰, 刘海龙, 伊港. 三氟甲烷磺酸钪催化醇对邻亚甲基苯醌的氧杂迈克尔加成反应[J]. 有机化学, 2019, 39(2): 555-560. |
| [11] | 郑楠, 宋汪泽. 过渡金属催化1,3-二羰基化合物不对称烯丙基烷基化反应的研究进展[J]. 有机化学, 2017, 37(5): 1099-1110. |
| [12] | 江晓莉, 戴伟, 赵佳佳, 石枫. 布朗斯特酸催化下邻羟基苯乙烯与吲哚的反应——1,1-二芳基乙烷类化合物的合成[J]. 有机化学, 2016, 36(5): 1014-1020. |
| [13] | 徐清, 李强. 过渡金属催化醇与胺有氧脱水反应及相关研究进展[J]. 有机化学, 2013, 33(01): 18-35. |
| [14] | 陆鸿飞, 孙垒垒, 武鼎铭, 高玉华, 石亚丽, 薛芹. 聚乙二醇酸性双子离子液体催化6-氨基取代嘌呤衍生物的合成及其抑菌活性[J]. 有机化学, 2012, 32(10): 1880-1887. |
| [15] | 高艳炫, 周宏勇, 李云庆, 王家喜. 氮杂环卡宾的合成及在氢转移反应中的应用[J]. 有机化学, 2012, 32(08): 1493-1497. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||