有机化学 ›› 2022, Vol. 42 ›› Issue (1): 277-292.DOI: 10.6023/cjoc202106053 上一篇 下一篇
研究论文
陈睿嘉a, 周聪a, 逄锡文a, 刘佳君b, 顾玉诚c, 刘建文b, 李忠a,*( )
)
收稿日期:2021-06-29
修回日期:2021-08-02
发布日期:2021-09-02
通讯作者:
李忠
基金资助:
Ruijia Chena, Cong Zhoua, Xiwen Panga, Jiajun Liub, Yucheng Guc, Jianwen Liub, Zhong Lia( )
)
Received:2021-06-29
Revised:2021-08-02
Published:2021-09-02
Contact:
Zhong Li
Supported by:文章分享

为了探索具有新型骨架的抗癌剂, 设计了一系列的以环丙烷限制构象的双酰胺类化合物. 大多数含有顺式环丙烷中心的外消旋体对四种肿瘤细胞系(MCF-7、BGC-823、HepG2和NCI-H460)具有良好的抑制活性. 其中, 消旋体顺式-N1-(2-甲基-4-(全氟丙-2-基)苯基)-N2-(4-苯氧基苄基)环丙烷-1,2-二羧酰胺(1-19)对MCF-7的IC50值为(8.38±0.67) mg•L–1, 显示出可进一步优化为抗癌先导物的潜力. 此外, 甾醇硫酸酯酶(steryl-sulfatase, STS)被PharmMapper预测为可能的靶标蛋白, 并由分子对接实验进行了验证.
陈睿嘉, 周聪, 逄锡文, 刘佳君, 顾玉诚, 刘建文, 李忠. 以环丙烷限制构象的新型双酰胺的设计、合成、抗癌活性及计算分析[J]. 有机化学, 2022, 42(1): 277-292.
Ruijia Chen, Cong Zhou, Xiwen Pang, Jiajun Liu, Yucheng Gu, Jianwen Liu, Zhong Li. Design, Synthesis, Anti-cancer Activities and Computational Analysis of Novel Diamides Conformationally Restricted by Cyclopropane[J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 277-292.
| Compd. | Structure (Relative stereochemistry) | Growth-inhibition (200 μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-2 | | 83.46±3.64 | 54.78±1.16 | 48.13±1.69 | 97.31±0.18 |
| 1-31 | | 19.42±1.19 | –28.92±2.03 | 38.24±5.37 | 14.20±6.58 |
| Compd. | Structure (Relative stereochemistry) | Growth-inhibition (200 μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-2 | | 83.46±3.64 | 54.78±1.16 | 48.13±1.69 | 97.31±0.18 |
| 1-31 | | 19.42±1.19 | –28.92±2.03 | 38.24±5.37 | 14.20±6.58 |
| Compd. | R1 | Growth-inhibition (200 μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-1 | Methyl | 37.23±3.34 | 32.39±1.17 | 23.47±4.79 | 47.37±0.50 |
| 1-2 | Ethyl | 83.46±3.64 | 54.78±1.16 | 48.13±1.69 | 97.31±0.18 |
| 1-3 | tert-Butyl | 85.73±2.92 | 4.17±0.52 | 57.57±1.57 | 94.34±2.25 |
| 1-4 | Benzyl | 96.83±0.69 | 97.75±0.08 | 99.14±0.28 | 98.42±0.16 |
| 1-30 | Phenethyl | 90.96±0.28 | 91.19±0.99 | 93.00±8.44 | 97.46±0.16 |
| Compd. | R1 | Growth-inhibition (200 μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-1 | Methyl | 37.23±3.34 | 32.39±1.17 | 23.47±4.79 | 47.37±0.50 |
| 1-2 | Ethyl | 83.46±3.64 | 54.78±1.16 | 48.13±1.69 | 97.31±0.18 |
| 1-3 | tert-Butyl | 85.73±2.92 | 4.17±0.52 | 57.57±1.57 | 94.34±2.25 |
| 1-4 | Benzyl | 96.83±0.69 | 97.75±0.08 | 99.14±0.28 | 98.42±0.16 |
| 1-30 | Phenethyl | 90.96±0.28 | 91.19±0.99 | 93.00±8.44 | 97.46±0.16 |
| Compd. | R1 | IC50/(μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-1 | Methyl | >200 | >200 | >200 | >200 |
| 1-2 | Ethyl | 33.81±2.06 | >200 | >200 | 29.80±2.91 |
| 1-3 | tert-Butyl | 29.27±5.83 | >200 | >200 | 38.38±1.40 |
| 1-4 | Benzyl | 40.79±2.18 | 50.90±0.99 | 26.09±2.97 | 26.78±2.78 |
| 1-5 | 4-Methylbenzyl | 32.29±1.7 | 21.25±3.03 | 10.44±1.60 | 20.38±1.21 |
| 1-6 | 3-Methylbenzyl | 40.35±5.19 | 35.50±3.97 | 35.01±1.54 | 30.59±1.18 |
| 1-7 | 2-Methylbenzyl | 33.80±1.72 | 25.42±2.29 | 23.20±1.07 | 21.60±1.60 |
| 1-8 | 4-Chlorobenzyl | 36.89±2.29 | 19.47±5.37 | 17.65±1.24 | 10.06±1.82 |
| 1-9 | 3-Chlorobenzyl | 36.80±4.39 | 30.58±2.65 | 28.62±0.81 | 22.48±0.71 |
| 1-10 | 2-Chlorobenzyl | 80.47±6.93 | 31.75±4.74 | 22.75±0.84 | 21.61±1.30 |
| 1-11 | 4-Fluorobenzyl | 67.45±2.43 | 29.55±2.70 | 27.07±1.05 | 25.27±2.85 |
| 1-12 | 4-Bromobenzyl | 17.65±0.99 | 28.29±2.54 | 21.17±0.81 | 21.01±1.25 |
| 1-13 | 4-Iodobenzyl | 27.57±6.26 | 26.24±2.70 | 26.29±1.43 | 26.15±1.36 |
| 1-14 | 4-Methoxybenzyl | 20.78±1.38 | 24.51±2.57 | 24.14±1.23 | 25.17±1.84 |
| 1-15 | 4-Difluoromethoxybenzyl | 75.61±14.80 | 50.03±2.97 | 39.52±6.27 | 56.80±10.58 |
| 1-16 | 4-tert-Butylbenzyl | >200 | 92.61±16.39 | >200 | 95.35±17.62 |
| 1-17 | 4-Cyanobenzyl | 47.75±9.06 | 42.49±4.12 | 37.92±2.94 | 30.26±3.15 |
| 1-18 | 4-Trifluoromethylbenzyl | 25.66±1.11 | 21.39±1.11 | 21.23±1.99 | 15.12±1.43 |
| 1-19 | (4-Phenoxyphenyl)methyl | 8.38±0.67 | 22.67±0.80 | 19.30±1.10 | 20.49±1.32 |
| 1-20 | 4-Phenylbenzyl | 25.88±2.95 | 24.97±1.27 | 27.06±3.07 | 22.94±1.15 |
| 1-21 | 2,4-Difluorobenzyl | 40.84±1.24 | 51.29±1.09 | 23.52±2.50 | 28.35±2.28 |
| 1-22 | 2,6-Dichlorobenzyl | >200 | >200 | 19.74±0.79 | 39.89±9.27 |
| 1-23 | 3,4,5-Trimethoxybenzyl | 19.16±3.01 | 34.39±3.52 | 27.22±2.61 | 20.39±1.41 |
| 1-24 | (Tetrahydrofuran-3-yl)methyl | 153.9±20.2 | 50.30±6.65 | 46.88±5.39 | 56.51±11.21 |
| 1-25 | (6-Chloropyridine-3-yl)methyl | 27.12±0.92 | 32.39±3.46 | 34.15±0.94 | 29.36±1.79 |
| 1-26 | (2-Chlorothiazole-5-yl)methyl | >200 | >200 | >200 | >200 |
| 1-27 | Furan-2-ylmethyl | 25.8±1.32 | 32.39±4.40 | 15.42±4.40 | 27.03±2.49 |
| 1-28 | Thiophene-3-ylmethyl | 83.43±4.37 | >200 | >200 | >200 |
| 1-29 | Naphthalene-1-ylmethyl | 32.53±2.82 | 22.91±1.20 | 23.55±1.97 | 20.52±0.98 |
| 1-30 | Phenethyl | 59.05±3.60 | 53.12±1.16 | 26.76±1.18 | 30.37±2.36 |
| 5-Fu | — | 8.23±1.33 | 10.31±1.40 | 7.09±1.61 | 2.26±0.80 |
| Compd. | R1 | IC50/(μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-1 | Methyl | >200 | >200 | >200 | >200 |
| 1-2 | Ethyl | 33.81±2.06 | >200 | >200 | 29.80±2.91 |
| 1-3 | tert-Butyl | 29.27±5.83 | >200 | >200 | 38.38±1.40 |
| 1-4 | Benzyl | 40.79±2.18 | 50.90±0.99 | 26.09±2.97 | 26.78±2.78 |
| 1-5 | 4-Methylbenzyl | 32.29±1.7 | 21.25±3.03 | 10.44±1.60 | 20.38±1.21 |
| 1-6 | 3-Methylbenzyl | 40.35±5.19 | 35.50±3.97 | 35.01±1.54 | 30.59±1.18 |
| 1-7 | 2-Methylbenzyl | 33.80±1.72 | 25.42±2.29 | 23.20±1.07 | 21.60±1.60 |
| 1-8 | 4-Chlorobenzyl | 36.89±2.29 | 19.47±5.37 | 17.65±1.24 | 10.06±1.82 |
| 1-9 | 3-Chlorobenzyl | 36.80±4.39 | 30.58±2.65 | 28.62±0.81 | 22.48±0.71 |
| 1-10 | 2-Chlorobenzyl | 80.47±6.93 | 31.75±4.74 | 22.75±0.84 | 21.61±1.30 |
| 1-11 | 4-Fluorobenzyl | 67.45±2.43 | 29.55±2.70 | 27.07±1.05 | 25.27±2.85 |
| 1-12 | 4-Bromobenzyl | 17.65±0.99 | 28.29±2.54 | 21.17±0.81 | 21.01±1.25 |
| 1-13 | 4-Iodobenzyl | 27.57±6.26 | 26.24±2.70 | 26.29±1.43 | 26.15±1.36 |
| 1-14 | 4-Methoxybenzyl | 20.78±1.38 | 24.51±2.57 | 24.14±1.23 | 25.17±1.84 |
| 1-15 | 4-Difluoromethoxybenzyl | 75.61±14.80 | 50.03±2.97 | 39.52±6.27 | 56.80±10.58 |
| 1-16 | 4-tert-Butylbenzyl | >200 | 92.61±16.39 | >200 | 95.35±17.62 |
| 1-17 | 4-Cyanobenzyl | 47.75±9.06 | 42.49±4.12 | 37.92±2.94 | 30.26±3.15 |
| 1-18 | 4-Trifluoromethylbenzyl | 25.66±1.11 | 21.39±1.11 | 21.23±1.99 | 15.12±1.43 |
| 1-19 | (4-Phenoxyphenyl)methyl | 8.38±0.67 | 22.67±0.80 | 19.30±1.10 | 20.49±1.32 |
| 1-20 | 4-Phenylbenzyl | 25.88±2.95 | 24.97±1.27 | 27.06±3.07 | 22.94±1.15 |
| 1-21 | 2,4-Difluorobenzyl | 40.84±1.24 | 51.29±1.09 | 23.52±2.50 | 28.35±2.28 |
| 1-22 | 2,6-Dichlorobenzyl | >200 | >200 | 19.74±0.79 | 39.89±9.27 |
| 1-23 | 3,4,5-Trimethoxybenzyl | 19.16±3.01 | 34.39±3.52 | 27.22±2.61 | 20.39±1.41 |
| 1-24 | (Tetrahydrofuran-3-yl)methyl | 153.9±20.2 | 50.30±6.65 | 46.88±5.39 | 56.51±11.21 |
| 1-25 | (6-Chloropyridine-3-yl)methyl | 27.12±0.92 | 32.39±3.46 | 34.15±0.94 | 29.36±1.79 |
| 1-26 | (2-Chlorothiazole-5-yl)methyl | >200 | >200 | >200 | >200 |
| 1-27 | Furan-2-ylmethyl | 25.8±1.32 | 32.39±4.40 | 15.42±4.40 | 27.03±2.49 |
| 1-28 | Thiophene-3-ylmethyl | 83.43±4.37 | >200 | >200 | >200 |
| 1-29 | Naphthalene-1-ylmethyl | 32.53±2.82 | 22.91±1.20 | 23.55±1.97 | 20.52±0.98 |
| 1-30 | Phenethyl | 59.05±3.60 | 53.12±1.16 | 26.76±1.18 | 30.37±2.36 |
| 5-Fu | — | 8.23±1.33 | 10.31±1.40 | 7.09±1.61 | 2.26±0.80 |
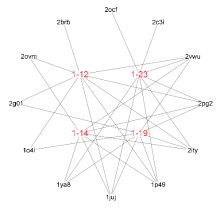
| Compd. | Pharma model (PDB ID) | Normalized fit score |
|---|---|---|
| 1-12 | 1p49 | 0.996 |
| 2vwu | 0.996 | |
| 2brb | 0.988 | |
| 2pg2 | 0.980 | |
| 1juj | 0.963 | |
| 2g01 | 0.956 | |
| 2ity | 0.935 | |
| 2ovm | 0.929 | |
| 1o4i | 0.906 | |
| 1-14 | 1p49 | 0.996 |
| 2vwu | 0.995 | |
| 2pg2 | 0.979 | |
| 1juj | 0.977 | |
| 1ya8 | 0.957 | |
| 2ovm | 0.944 | |
| 2ity | 0.925 | |
| 1-19 | 1p49 | 0.996 |
| 2vwu | 0.994 | |
| 2pg2 | 0.980 | |
| 1juj | 0.971 | |
| 1ya8 | 0.971 | |
| 2g01 | 0.946 | |
| 2ity | 0.928 | |
| 1o4i | 0.903 | |
| 1-23 | 1p49 | 0.995 |
| 2vwu | 0.993 | |
| 2c3i | 0.987 | |
| 2pg2 | 0.979 | |
| 1ya8 | 0.972 | |
| 1juj | 0.967 | |
| 2ocf | 0.942 | |
| 2ity | 0.930 |
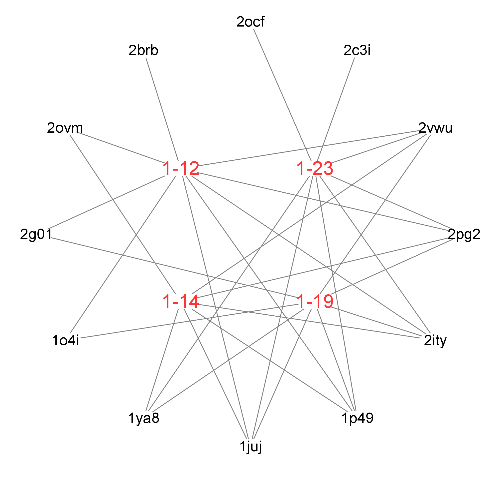
| Compd. | Pharma model (PDB ID) | Normalized fit score |
|---|---|---|
| 1-12 | 1p49 | 0.996 |
| 2vwu | 0.996 | |
| 2brb | 0.988 | |
| 2pg2 | 0.980 | |
| 1juj | 0.963 | |
| 2g01 | 0.956 | |
| 2ity | 0.935 | |
| 2ovm | 0.929 | |
| 1o4i | 0.906 | |
| 1-14 | 1p49 | 0.996 |
| 2vwu | 0.995 | |
| 2pg2 | 0.979 | |
| 1juj | 0.977 | |
| 1ya8 | 0.957 | |
| 2ovm | 0.944 | |
| 2ity | 0.925 | |
| 1-19 | 1p49 | 0.996 |
| 2vwu | 0.994 | |
| 2pg2 | 0.980 | |
| 1juj | 0.971 | |
| 1ya8 | 0.971 | |
| 2g01 | 0.946 | |
| 2ity | 0.928 | |
| 1o4i | 0.903 | |
| 1-23 | 1p49 | 0.995 |
| 2vwu | 0.993 | |
| 2c3i | 0.987 | |
| 2pg2 | 0.979 | |
| 1ya8 | 0.972 | |
| 1juj | 0.967 | |
| 2ocf | 0.942 | |
| 2ity | 0.930 |
| [1] |
Torre, L. A.; Bray, F.; Siegel, R. L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. CA-Cancer J. Clin. 2015, 65, 87.
doi: 10.3322/caac.21262 |
| [2] |
Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. CA-Cancer J. Clin. 2021, 71, 209.
doi: 10.3322/caac.v71.3 |
| [3] |
Dickens, E.; Ahmed, S. Surgery (Oxford) 2018, 36, 134.
doi: 10.1016/j.mpsur.2017.12.002 |
| [4] |
Jagoe, K. N. R. T.; Abalo, R. Front. Pharmacol. 2018, 9, 245.
doi: 10.3389/fphar.2018.00245 |
| [5] |
Janganati, V.; Ponder, J.; Thakkar, S.; Thakkar, S.; Jordan, C. T.; Crooks, P. A. Bioorg. Med. Chem. 2017, 25, 3694.
|
| [6] |
Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471.
doi: 10.1038/nature10702 |
| [7] |
Kumari, S.; Carmona, A. V.; Tiwari, A. K.; Trippier, P. C. J. Med. Chem. 2020, 63, 12290
doi: 10.1021/acs.jmedchem.0c00530 |
| [8] |
Vlaar, C. P.; Castillo-Pichardo, L.; Medina, J. I.; Marrero-Serra, C. M.; Vélez, E; Ramos, Z.; Hernández, E. Bioorg. Med. Chem. 2018, 26, 884.
doi: 10.1016/j.bmc.2018.01.003 |
| [9] |
Rai, U. S.; Isloor, A. M.; Shetty, P.; Pai, K. S. R.; Fun, H. K. Arabian J. Chem. 2015, 8, 317.
doi: 10.1016/j.arabjc.2014.01.018 |
| [10] |
Shao, P. P.; Ok, D.; Fisher, M. H.; Garcia, M. L.; Kaczorowski, G. J.; Li, C.; Lyons, K. A.; Martin, W. J.; Meinke, P. T.; Priest, B. T.; Smith, M. M.; Wyvratt, M. J.; Ye, F.; Parsons, W. H. Bioorg. Med. Chem. Lett. 2005, 15, 1901.
doi: 10.1016/j.bmcl.2005.02.002 |
| [11] |
Tarzia, G.; Shiatti, P.; Selva, D.; Favara, D.; Ceriani, S. Eur. J. Med. Chem. 1976, 11, 263.
|
| [12] |
Hickey, S. M.; Ashton, T. D.; Khosa, S. K.; Robson, R. N.; White, J. M.; Li, J.; Nation, R. L.; Yu, H. Y.; Elliott, A. G.; Butler, M. S.; Huang, J. X.; Cooper, M. A.; Pfeffer, F. M Org. Biomol. Chem. 2015, 22, 6255.
|
| [13] |
Saudi, M.; Zmurko, J.; Kaptein, S.; Rpzenski, J.; Gadakh, B.; Chaltin, P.; Marchand, A.; Neyts, J.; Aerschot, A. V. Eur. J. Med. Chem. 2016, 12, 158.
|
| [14] |
Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. J. Pestic. Sci. 2005, 30, 354.
doi: 10.1584/jpestics.30.354 |
| [15] |
Galano, A.; Alvarez-Idaboy, J. R.; Vivier-Bunge, A. Theor. Chem. Acc. 2007, 118, 597.
doi: 10.1007/s00214-007-0353-z |
| [16] |
Talele, T. T. J. Med. Chem. 2016, 59, 8712.
doi: 10.1021/acs.jmedchem.6b00472 |
| [17] |
Julius, D. A. Am. J. Psychiatry 1979, 136, 782.
doi: 10.1176/ajp.136.6.782 |
| [18] |
Yakes, F. M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; Orf, J.; You, A.; Laird, A. D.; Engst, S.; Lee, L.; Lesch, J.; Chou, Y.-C.; Joly, A. H. J. Clin. Oncol. 2017, 35, 591.
doi: 10.1200/JCO.2016.70.7398 |
| [19] |
Chopra, N.; Nathan, P. D. Expert Rev. Anticancer Ther. 2015, 15, 749.
doi: 10.1586/14737140.2015.1060127 |
| [20] |
Scheciiter, M. S.; Sullivan, W. N.; Schoof, H. F.; Maddock, D. R.; Amyx, C. M.; Porter, J. E. J. Med. Entomol. 1974, 11, 231.
pmid: 4851258 |
| [21] |
Matsui, K.; Kido, Y.; Watari, R.; Kashima, Y.; Yoshida, Y.; Shuto, S. Chem. Eur. J. 2017, 23, 3034.
doi: 10.1002/chem.201604946 |
| [22] |
Lavedan, C.; Forsberg, M.; Gentile, A. J. Neuropharmacology 2015, 91, 142.
doi: 10.1016/j.neuropharm.2014.12.004 |
| [23] |
Yamaguchi, K.; Kazuta, Y.; Hirano, K.; Yamada, S.; Matsuda, A.; Shuto, S. Bioorg. Med. Chem. 2008, 16, 8875.
doi: 10.1016/j.bmc.2008.08.061 |
| [24] |
Martin, S. F.; Dorsey, G. O.; Gane, T.; Hillier, M. C.; Kessler, H.; Baur, M.; Mathä, B.; Erickson, J. W.; Bhat, T. N.; Munshi, S.; Gulnik, S. V.; Topol, I. A. J. Med. Chem. 1998, 41, 1581.
pmid: 9572884 |
| [25] |
Chen, Y.; Le, V.; Xu, X.; Shao, X.; Liu, J.; Li, Z. Bioorg. Med. Chem. Lett. 2014, 24, 3948.
doi: 10.1016/j.bmcl.2014.06.041 |
| [26] |
Ozoe, Y.; Kita, T.; Ozoe, F.; Nakao, T.; Sato, K.; Hirase, K. Pestic. Biochem. Physiol. 2013, 107, 285.
doi: 10.1016/j.pestbp.2013.09.005 |
| [27] |
Shang, J.; Liu, Q.; Wang, B.; Li, Z. Chin. J. Org. Chem. 2019, 39, 1489. (in Chinese)
doi: 10.6023/cjoc201810025 |
|
(尚俊峰, 刘巧霞, 王宝雷, 李正名, 有机化学, 2019, 39, 1489.)
doi: 10.6023/cjoc201810025 |
|
| [28] |
Chen, K.; Liu, Q.; Ni, J.; Zhu, H.; Li, Y.; Wang, Q. Pest Manage. Sci. 2014, 71, 1503.
doi: 10.1002/ps.3954 |
| [29] |
Duan, J. J.-W.; Lu, Z.; Jiang, B.; Stachura, S.; Weigelt, C. A.; Sack, J. S.; Khan, J.; Ruzanov, M.; Galella, M. A.; Wu, D.-R.; Yarde, M.; Shen, D.-R.; Shuster, D. J.; Borowski, V.; Xie, J. H.; Zhang, L.; Vanteru, S.; Gupta, A. K.; Mathur, A.; Zhao, Q.; Foster, W.; Salter-Cid, L. M.; Carter, P. H.; Dhar, T. G. M. ACS Med. Chem. Lett. 2019, 10, 367.
doi: 10.1021/acsmedchemlett.9b00010 |
| [30] |
Li, Z.; Xu, X.; Chen, Y.; Liu, J.; Li, W. CN 103435562, 2013. (in Chinese)
|
| [31] |
Li, Z.; Chen, Y.; Liu, A.; Li, Y.; Wang, B.; Pan, L.; Wan, Y.; Liu, J.; Chen, W. CN 104402785, 2015. (in Chinese)
|
| [32] |
Lepri, S.; Goracci, L.; Valeri, A.; Cruciani, G. Eur. J. Med. Chem. 2016, 121, 658.
doi: 10.1016/j.ejmech.2016.06.006 |
| [33] |
Simpkins, L. M.; Bolton, S.; Pi, Z.; Sutton, J. C.; Kwon, C.; Zhao, G.; Magnin, D. R.; Augeri, D. J.; Gungor, T.; Rotella, D. P.; Sun, Z.; Liu, Y.; Slusarchyk, W. S.; Marcinkeviciene, J.; Robertson, J. G.; Wang, A.; Robl, J. A.; Atwal, K. S.; Zahler, R. L.; Parker, R. A.; Kirby, M. S.; Hamann, L. G. Bioorg. Med. Chem. Lett. 2007, 17, 6476.
pmid: 17937986 |
| [34] |
Wang, F.; Xu, X. Wang, F; Peng, L.; Zhang, Y.; Wang, L.; Wang, L. Lett. Org. Chem. 2015, 12, 741.
doi: 10.2174/1570178612666150907204813 |
| [35] |
Milewska, M. J.; Gdaniec, M.; Poloński, T. Tetrahedron: Asymmetry 1996, 7, 3169.
doi: 10.1016/0957-4166(96)00419-3 |
| [36] |
Beutner, G. L.; Young, I. S.; Davies, M. L.; Hickey, M. R.; Park, H.; Stevens, J. M.; Ye, Q. Org. Lett. 2018, 20, 4218.
doi: 10.1021/acs.orglett.8b01591 |
| [37] |
Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. Nucleic Acids Res. 2010, 38, 609.
|
| [38] |
Wang, X.; Pan, C.; Gong, J.; Liu, X.; Li, H. J. Chem. Inf. Model. 2016, 56, 1175.
doi: 10.1021/acs.jcim.5b00690 pmid: 27187084 |
| [39] |
Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. Nucleic Acids Res. 2017, 45, 356.
doi: 10.1093/nar/gkx374 pmid: 28472422 |
| [40] |
Ferrante, P.; Messali, S.; Meroni, G.; Ballabio, A. Eur. J. Hum. Genet. 2002, 10, 813.
doi: 10.1038/sj.ejhg.5200887 pmid: 12461688 |
| [41] |
Hernandez-Guzman, F. G.; Higashiyama, T.; Pangborn, W.; Osawa, Y.; Ghosh, D. J. Biol. Chem. 2003, 278, 22989.
pmid: 12657638 |
| [42] |
Geisler, J.; Sasano, H.; Chen, S.; Purohit, A. J. Steroid Biochem. Mol. Biol. 2011, 125, 39.
doi: 10.1016/j.jsbmb.2011.02.002 |
| [43] |
Purohit, A.; Foster, P. A. J. Endocrinol. 2012, 212, 99.
doi: 10.1530/JOE-11-0266 pmid: 21859802 |
| [44] |
Leese, M. P.; Hejaz, H. A. M.; Mahon, M. F.; Newman, S. P.; Purohit, A.; Reed, M. J.; Potter, B. V. L. J. Med. Chem. 2005, 48, 5243.
doi: 10.1021/jm050066a |
| [45] |
A. Purohit, Woo, L. W. L.; Potter, B. V. L. Cancer Res. 2000, 60, 3394.
pmid: 10910045 |
| [46] |
Kertesz, N.; Krasnoperov, V.; Reddy, R.; Leshanski, L.; Kumar, S. R.; Zozulya, S.; Gill, P. S. Blood 2006, 107, 2330.
doi: 10.1182/blood-2005-04-1655 |
| [47] |
Pasquale, E. B. Nat. Rev. Cancer 2010, 10, 165.
doi: 10.1038/nrc2806 pmid: 20179713 |
| [48] |
Dohle, W.; Jourdan, F. L.; Menchon, G.; Prota, A. E.; Foster, P. A.; Mannion, P.; Hamel, E.; Thomas, M. P.; Kasprzyk, P. G.; Ferrandis, E.; Steinmetz, M. O.; Leese, M. P.; Potter, B. V. L. J. Med. Chem. 2018, 61, 1031.
doi: 10.1021/acs.jmedchem.7b01474 |
| [49] |
Maltais, R.; Djiemeny, A. N.; Roy, J.; Barbeau, X.; Lambert, J.-P.; Poirier, D. Bioorg. Med. Chem. 2020, 28, 115368.
doi: 10.1016/j.bmc.2020.115368 |
| [1] | 吴思敏, 唐嘉欣, 周于佳, 徐学涛, 张昊星, 王少华. 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024, 44(2): 613-621. |
| [2] | 陈祖良, 魏颖静, 张俊良. 供体-受体氮杂环丙烷碳-碳键断裂的环加成反应研究进展[J]. 有机化学, 2023, 43(9): 3078-3088. |
| [3] | 王锋, 陈钰, 裴鸿艳, 张静, 张立新. 含哌啶的新型1,2,4-噁二唑类衍生物的设计合成及抗真菌活性研究[J]. 有机化学, 2023, 43(8): 2826-2836. |
| [4] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [5] | 钟玉梅, 邹小颖, 卓小丫, 王逸涵, 申佳奕, 郑绿茵, 郭维. 4-氧代-2-亚胺基噻唑烷-5-亚基乙酸乙酯类化合物的设计、合成及抗癌活性[J]. 有机化学, 2023, 43(4): 1452-1461. |
| [6] | 陈文龙, 李慧敏, 杨鹏飞, 郑东程, 杨高升. 2-芳甲酰基甲亚基丙二酸酯与Corey叶立德的反应[J]. 有机化学, 2023, 43(4): 1472-1482. |
| [7] | 梁志鹏, 叶浩, 张海滨, 姜国民, 吴新星. 环丁酮类腙参与的偕二氟环丙烷开环胺化反应[J]. 有机化学, 2023, 43(4): 1483-1491. |
| [8] | 刘悦灵, 钟欣欣, 张干兵. Pd(0)催化1-R-3-苯基亚丙基环丙烷(R=Me/H)与呋喃甲醛[3+2]环加成反应机理的密度泛函理论研究[J]. 有机化学, 2023, 43(2): 660-667. |
| [9] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [10] | 郝二军, 丁笑波, 王珂新, 周红昊, 杨启亮, 石磊. 氮杂环丙烷与不饱和化合物发生[3+2]扩环反应的研究进展[J]. 有机化学, 2023, 43(12): 4057-4074. |
| [11] | 孙昌兴, 张福豪, 张欢, 李鹏辉, 姜林. 新型2-(1-甲基-1H-吡唑-4-基)嘧啶-4-甲酰胺的设计、合成、杀菌活性及分子对接研究[J]. 有机化学, 2023, 43(1): 229-235. |
| [12] | 赵静, 金辄, 王润, 张新庚, 韩英妹, 胡春, 刘晓平, 张传明, 金丽萍. 2-[(吡啶-2-基甲基)硫基]-1H-苯并咪唑类化合物的设计、合成和抗癌活性研究[J]. 有机化学, 2022, 42(7): 2172-2183. |
| [13] | 胡冬燕, 韩广田, 李喜安, 任华忠, 岳李荣, 郭力, 封家福. 新型哈尔碱衍生物的合成与体外抗肿瘤活性研究[J]. 有机化学, 2022, 42(6): 1863-1871. |
| [14] | 王长凯, 孙腾达, 张学博, 杨新玲, 路星星, 徐欢, 石发胜, 张莉, 凌云. 新型含氟吡唑酰肼类化合物的设计合成与生物活性研究[J]. 有机化学, 2022, 42(5): 1527-1536. |
| [15] | 王秀, 段文贵, 林桂汕, 李宝谕, 张文静, 雷福厚. 含天然蒎烯结构的4-酰基-3-氨基-1,2,4-三唑-硫醚衍生物的合成、抑菌活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2022, 42(3): 871-883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||