有机化学 ›› 2022, Vol. 42 ›› Issue (3): 679-697.DOI: 10.6023/cjoc202110009 上一篇 下一篇
综述与进展
收稿日期:2021-10-09
修回日期:2021-11-13
发布日期:2021-11-25
通讯作者:
杨晓瑜
基金资助:
Yunrong Chen, Wei Liu, Xiaoyu Yang( )
)
Received:2021-10-09
Revised:2021-11-13
Published:2021-11-25
Contact:
Xiaoyu Yang
Supported by:文章分享

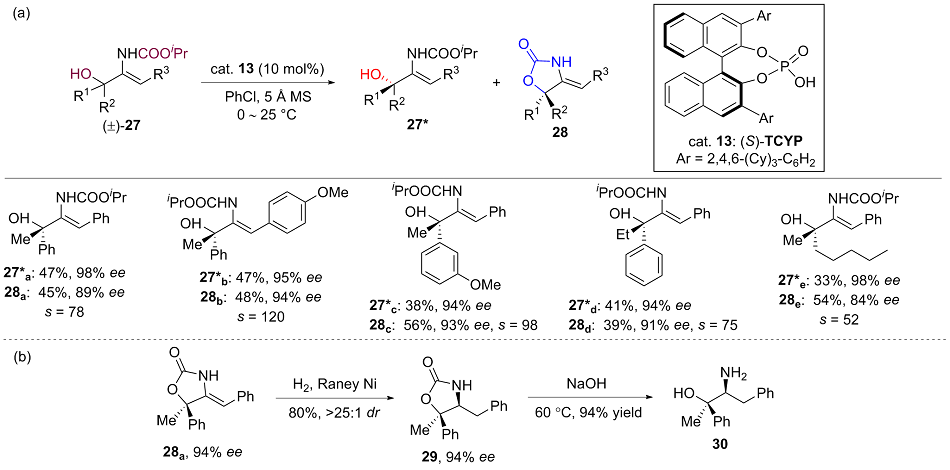
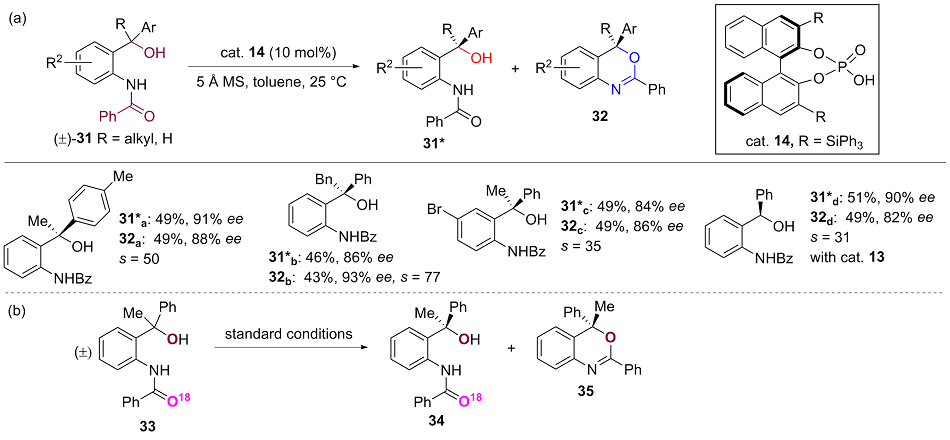
手性叔醇结构广泛存在于生物活性物质、天然产物和药物分子中, 实现其高效不对称催化合成具有重要意义. 消旋醇的动力学拆分是一种合成高光学纯度手性醇的重要方法, 然而由于叔醇α-碳上带三个不同的非氢取代基团, 手性识别难度较大, 因而发展高效且具有广泛底物适用性的叔醇动力学拆分方法具有较大的挑战. 尽管如此, 近年来非酶催化的叔醇的动力学拆分领域取得了快速的发展, 一些新颖的不对称催化反应、催化体系被成功应用于叔醇的动力学拆分反应中. 对叔醇动力学拆分反应进行了系统总结, 分类介绍了这些反应的底物适用性、特点、机理以及局限等, 并对该领域的未来发展进行展望.
陈运荣, 刘炜, 杨晓瑜. 叔醇的动力学拆分研究进展[J]. 有机化学, 2022, 42(3): 679-697.
Yunrong Chen, Wei Liu, Xiaoyu Yang. Recent Advances in Kinetic Resolution of Tertiary Alcohols[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 679-697.

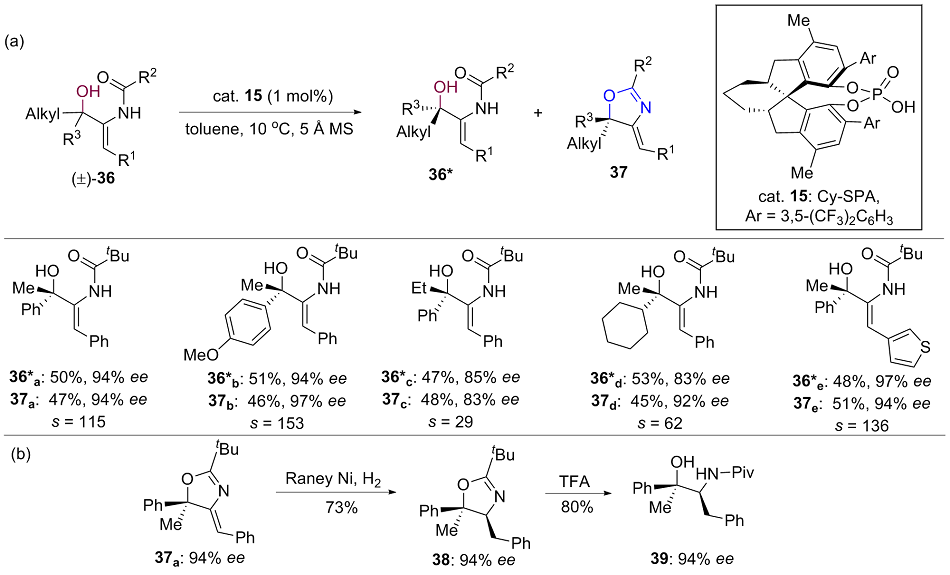

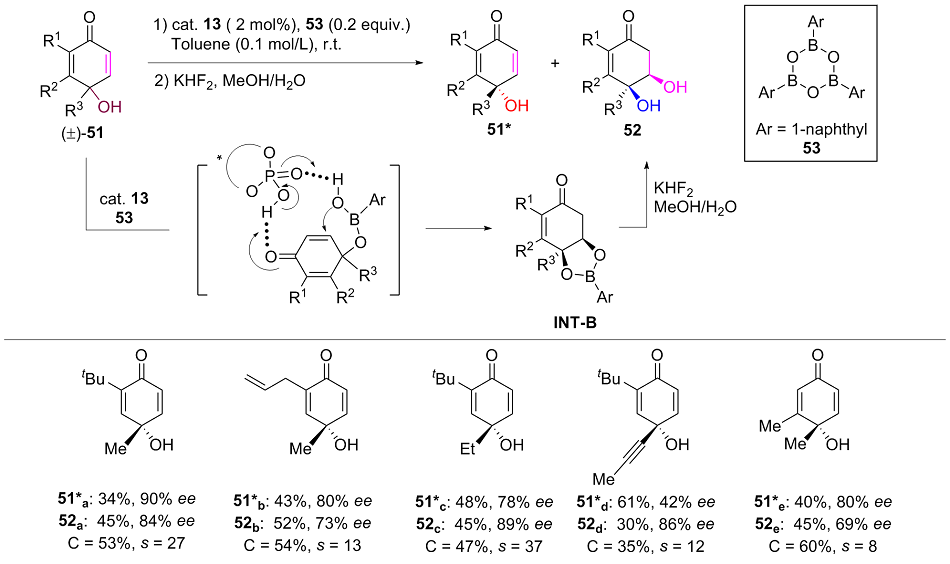

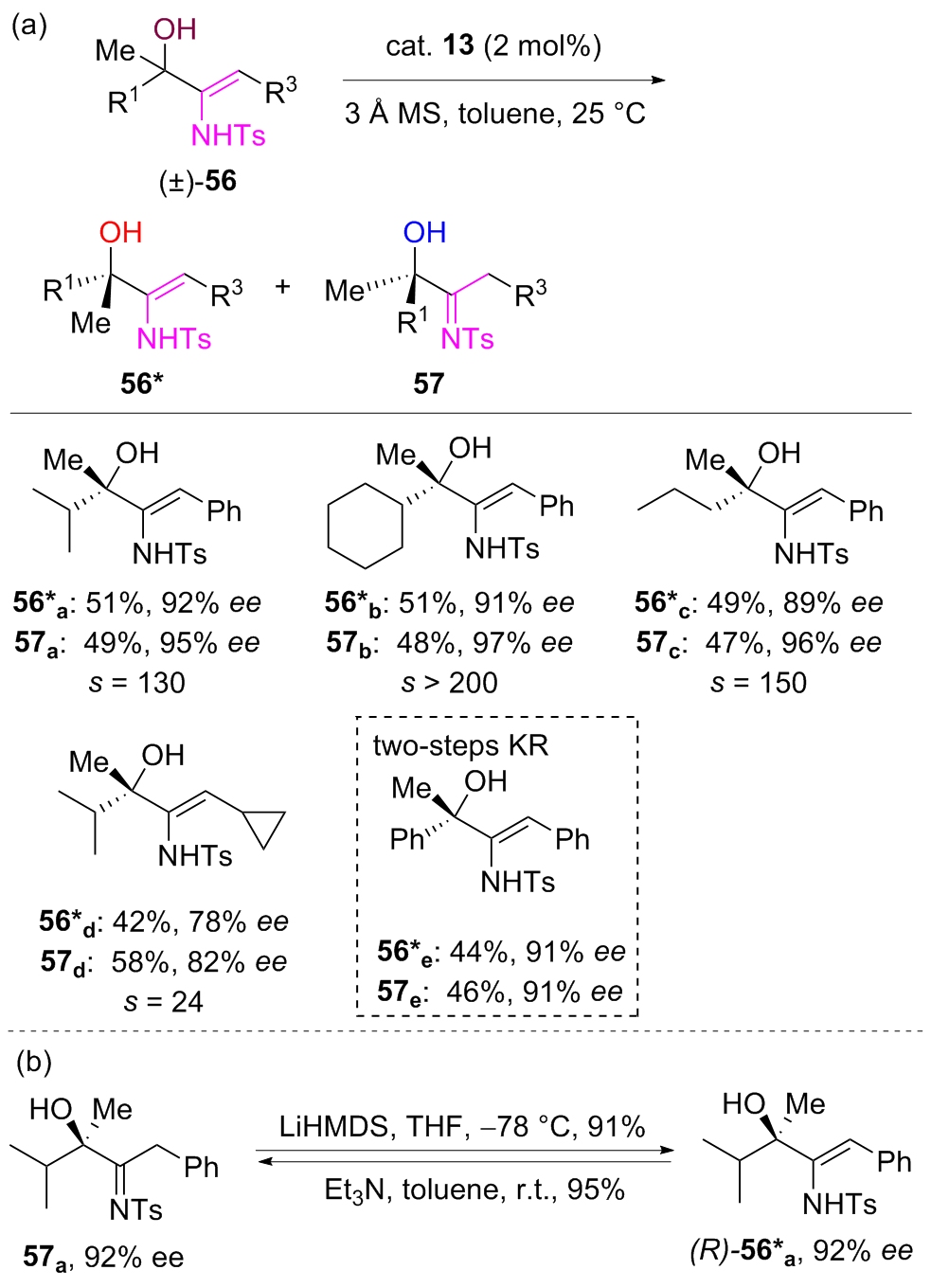
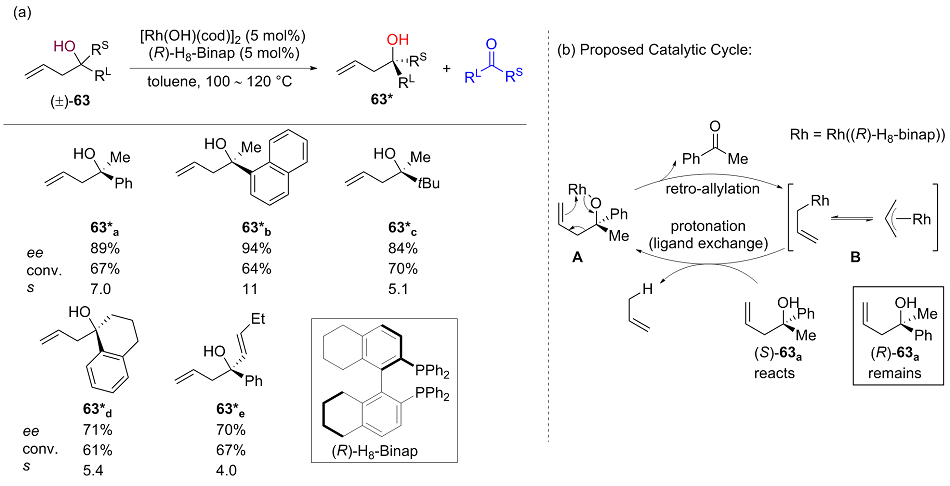

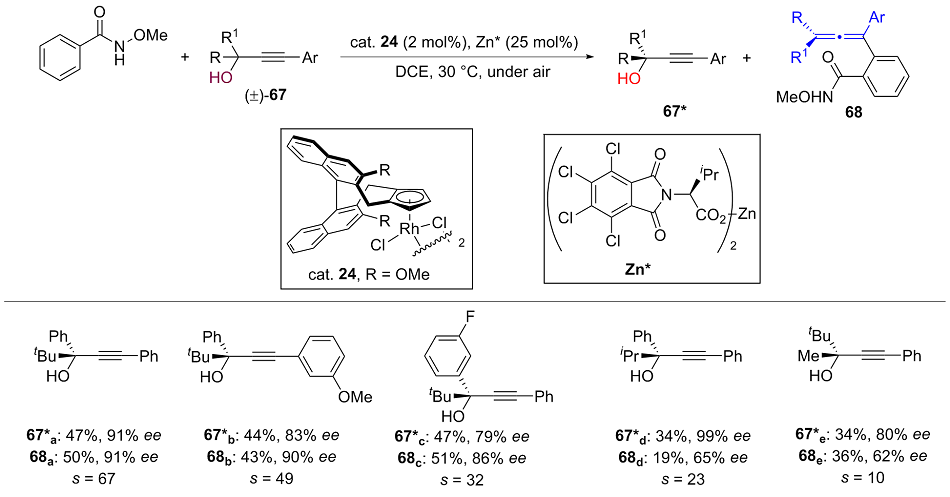
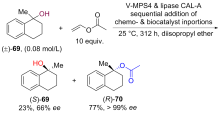
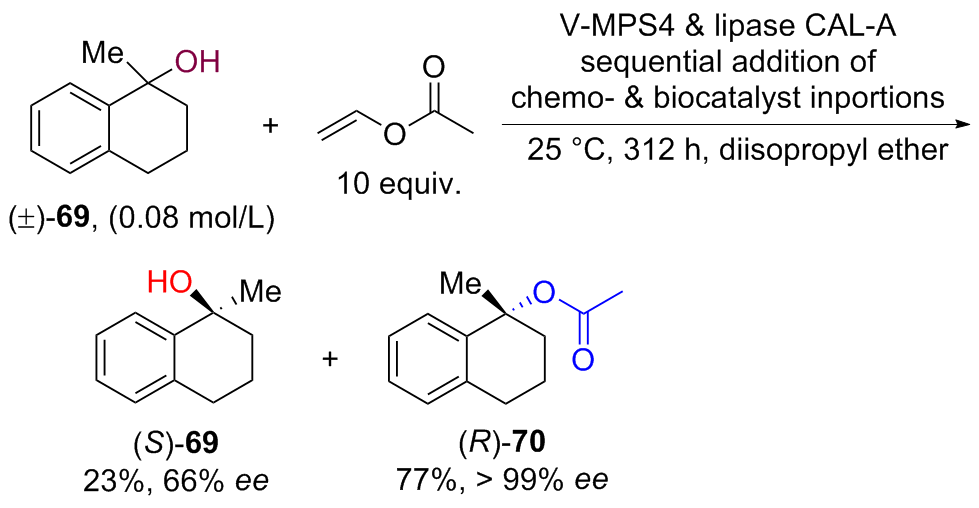
| [1] |
(a) Shibasaki, M.; Kanai, M. Chem. Rev. 2008, 108 (8), 2853.
doi: 10.1021/cr078340r |
|
(b) Liu, Y.-L.; Lin, X.-T. Adv. Syn. Catal. 2019, 361 (5), 876.
doi: 10.1002/adsc.v361.5 |
|
|
(c) Collados, J. F.; Solà, R.; Harutyunyan, S. R.; Maciá, B. ACS Catal. 2016, 6 (3), 1952.
doi: 10.1021/acscatal.5b02832 |
|
|
(d) Rong, J.; Pellegrini, T.; Harutyunyan, S. R. Chem.-Eur. J. 2016, 22 (11), 3558.
doi: 10.1002/chem.201503412 |
|
|
(e) Zhu, D.; Xu, M.-H. Chin. J. Org. Chem. 2020, 40, 255. (in Chinese)
doi: 10.6023/cjoc201910009 |
|
|
(祝东星, 徐明华, 有机化学, 2020, 40, 255.)
|
|
|
(f) Luo, R.; Liao, J.; Zhang, J. Chin. J. Org. Chem. 2013, 33, 2298. (in Chinese)
doi: 10.6023/cjoc201305044 |
|
|
(罗人仕, 廖建华, 张剑, 有机化学, 2013, 33, 2298.)
|
|
|
(g) Fu, Y.; Hou, B.; Zhao, X.; Du, Z.; Hu, Y. Chin. J. Org. Chem. 2015, 35, 2507. (in Chinese)
doi: 10.6023/cjoc201505045 |
|
|
(傅颖, 侯博, 赵兴玲, 杜正银, 胡雨来, 有机化学, 2015, 35, 2507.)
doi: 10.6023/cjoc201505045 |
|
| [2] |
(a) Sim, S.-B. D.; Wang, M.; Zhao, Y. ACS Catal. 2015, 5(6), 3609.
doi: 10.1021/acscatal.5b00583 |
|
(b) Bergonzini, G.; Melchiorre, P. Angew. Chem. Int. Ed. 2012, 51(4), 971.
doi: 10.1002/anie.v51.4 |
|
|
(c) Jung, B.; Hong, M. S.; Kang, S. H. Angew. Chem. Int. Ed. 2007, 46(15), 2616.
doi: 10.1002/(ISSN)1521-3773 |
|
| [3] |
(a) Robinson, D. E. J. E.; Bull, S. D. Tetrahedron: Asymmetry 2003, 14(11), 1407.
doi: 10.1016/S0957-4166(03)00209-X |
|
(b) Vedejs, E.; Jure, M. Angew. Chem. Int. Ed. 2005, 44(26), 3974.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(c) Müller, C. E.; Schreiner, P. R. Angew. Chem. Int. Ed. 2011, 50(27), 6012.
doi: 10.1002/anie.v50.27 |
|
|
(d) Pellissier, H. Adv. Syn. Catal. 2011, 353(10), 1613.
doi: 10.1002/adsc.201100111 |
|
|
(e) Krasnov, V. P.; Gruzdev, D. A.; Levit, G. L. Eur. J. Org. Chem. 2012, 2012(8), 1471.
doi: 10.1002/ejoc.v2012.8 |
|
|
(f) Petersen, K. S. Asian J. Org. Chem. 2016, 5(3), 308.
doi: 10.1002/ajoc.v5.3 |
|
|
(g) Chen, S.; Shi, Y.-H.; Wang, M. Chem. Asian J. 2018, 13(17), 2184.
doi: 10.1002/asia.201800537 |
|
|
(h) Wang, Z.; Pan, D.; Li, T.; Jin, Z. Chem. Asian J. 2018, 13(17), 2149.
doi: 10.1002/asia.201800493 |
|
|
(i) Yang, H.; Zheng, W.-H. Tetrahedron Lett. 2018, 59(7), 583.
doi: 10.1016/j.tetlet.2017.12.080 |
|
|
(j) Liu, W.; Yang, X. Asian J. Org. Chem. 2021, 10(4), 692.
doi: 10.1002/ajoc.v10.4 |
|
| [4] |
Kagna, H. B.; Fiaud, J. C., Topics in Stereochemistry. In Kinetic Resolution, Eliel, E. L.; Wilen, S. H., Eds. Wiley; New York: 1988; Vol. 18, p. 249.
|
| [5] |
(a) Özdemirhan, D. Synth. Commun. 2017, 47(7), 629.
doi: 10.1080/00397911.2016.1274032 |
|
(b) Özdemirhan, D.; Sezer, S.; Sönmez, Y. Tetrahedron: Asymmetry 2008, 19(23), 2717.
doi: 10.1016/j.tetasy.2008.12.002 |
|
|
(c) Deng, D.; Zhang, Y.; Sun, A.; Sai, K.; Hu, Y. Chin. J. Org. Chem. 2018, 38, 1185. (in Chinese)
doi: 10.6023/cjoc201710019 |
|
|
(邓盾, 张云, 孙爱君, 赛克, 胡云峰, 有机化学, 2018, 38, 1185.)
doi: 10.6023/cjoc201710019 |
|
| [6] |
Jarvo, E. R.; Evans, C. A.; Copeland, G. T.; Miller, S. J. J. Org. Chem. 2001, 66(16), 5522.
pmid: 11485477 |
| [7] |
Angione, M. C.; Miller, S. J. Tetrahedron 2006, 62(22), 5254.
doi: 10.1016/j.tet.2006.01.104 |
| [8] |
(a) Lu, S.; Poh, S. B.; Siau, W.-Y.; Zhao, Y. Angew. Chem., nt. Ed. 2013, 52(6), 1731.
|
|
(b) Lu, S.; Poh, S. B.; Siau, W.-Y.; Zhao, Y. Synlett 2013, 24(10), 1165.
doi: 10.1055/s-00000083 |
|
| [9] |
Greenhalgh, M. D.; Smith, S. M.; Walden, D. M.; Taylor, J. E.; Brice, Z.; Robinson, E. R. T.; Fallan, C.; Cordes, D. B.; Slawin, A. M. Z.; Richardson, H. C.; Grove, M. A.; Cheong, P. H.-Y.; Smith, A. D. Angew. Chem. Int. Ed. 2018, 57(12), 3200.
doi: 10.1002/anie.v57.12 |
| [10] |
Guha, N. R.; Neyyappadath, R. M.; Greenhalgh, M. D.; Chisholm, R.; Smith, S. M.; McEvoy, M. L.; Young, C. M.; Rodríguez- Escrich, C.; Pericàs, M. A.; Hähner, G.; Smith, A. D. Green Chem. 2018, 20(19), 4537.
doi: 10.1039/C8GC02020E |
| [11] |
Young, C. M.; Elmi, A.; Pascoe, D. J.; Morris, R. K.; McLaughlin, C.; Woods, A. M.; Frost, A. B.; de la Houpliere, A.; Ling, K. B.; Smith, T. K.; Slawin, A. M. Z.; Willoughby, P. H.; Cockroft, S. L.; Smith, A. D. Angew. Chem. Int. Ed. 2020, 59(9), 3705.
doi: 10.1002/anie.v59.9 |
| [12] |
Qu, S.; Smith, S. M.; Laina-Martín, V.; Neyyappadath, R. M.; Greenhalgh, M. D.; Smith, A. D. Angew. Chem. Int. Ed. 2020, 59(38), 16572.
doi: 10.1002/anie.v59.38 |
| [13] |
Mandai, H.; Shiomoto, R.; Fujii, K.; Mitsudo, K.; Suga, S. Org. Lett. 2021, 23(4), 1169.
doi: 10.1021/acs.orglett.0c03956 |
| [14] |
Karatas, B.; Rendler, S.; Fröhlich, R.; Oestreich, M. Org. Biomol. Chem. 2008, 6(8), 1435.
doi: 10.1039/b802186d pmid: 18385850 |
| [15] |
Seliger, J.; Dong, X.; Oestreich, M. Angew. Chem. Int. Ed. 2019, 58(7), 1970.
doi: 10.1002/anie.v58.7 |
| [16] |
Čorić, I.; Müller, S.; List, B. J. Am. Chem. Soc. 2010, 132(49), 17370.
doi: 10.1021/ja108642s |
| [17] |
Yamanaka, T.; Kondoh, A.; Terada, M. J. Am. Chem. Soc. 2015, 137(3), 1048.
doi: 10.1021/ja512238n pmid: 25581575 |
| [18] |
Kim, J. H.; Čorić, I.; Palumbo, C.; List, B. J. Am. Chem. Soc. 2015, 137(5), 1778.
doi: 10.1021/ja512481d |
| [19] |
Yoneda, N.; Matsumoto, A.; Asano, K.; Matsubara, S. Chem. Lett. 2016, 45(11), 1300.
doi: 10.1246/cl.160727 |
| [20] |
Rajkumar, S.; He, S.; Yang, X. Angew. Chem. Int. Ed. 2019, 58(30), 10315.
doi: 10.1002/anie.v58.30 |
| [21] |
Rajkumar, S.; Tang, M.; Yang, X. Angew. Chem. Int. Ed. 2020, 59(6), 2333.
doi: 10.1002/anie.v59.6 |
| [22] |
Pan, Y.; Jiang, Q.; Rajkumar, S.; Zhu, C.; Xie, J.; Yu, S.; Chen, Y.; He, Y. P.; Yang, X. Adv. Syn. Catal. 2020, 363(1), 200.
doi: 10.1002/adsc.v363.1 |
| [23] |
Zheng, Z.; Cao, Y.; Chong, Q.; Han, Z.; Ding, J.; Luo, C.; Wang, Z.; Zhu, D.; Zhou, Q. L.; Ding, K. J. Am. Chem. Soc. 2018, 140(32), 10374.
doi: 10.1021/jacs.8b07125 |
| [24] |
Zhang, C. H.; Gao, Q.; Li, M.; Wang, J. F.; Yu, C. M.; Mao, B. Org. Lett. 2021, 23(10), 3949.
doi: 10.1021/acs.orglett.1c01110 |
| [25] |
Hua, Y.; Liu, Z.-S.; Xie, P.-P.; Ding, B.; Cheng, H.-G.; Hong, X.; Zhou, Q. Angew. Chem. Int. Ed. 2021, 60(23), 12824.
doi: 10.1002/anie.v60.23 |
| [26] |
Schipper, D. J.; Rousseaux, S.; Fagnou, K. Angew. Chem. Int. Ed. 2009, 48(44), 8343.
doi: 10.1002/anie.200902373 |
| [27] |
Zhao, Y.; Mitra, A. W.; Hoveyda, A. H.; Snapper, M. L. Angew. Chem. Int. Ed. 2007, 46(44), 8471.
doi: 10.1002/(ISSN)1521-3773 |
| [28] |
Olivares-Romero, J. L.; Li, Z.; Yamamoto, H. J. Am. Chem. Soc. 2013, 135(9), 3411.
doi: 10.1021/ja401182a pmid: 23406082 |
| [29] |
Pawliczek, M.; Hashimoto, T.; Maruoka, K. Chem. Sci. 2018, 9(5), 1231.
doi: 10.1039/c7sc04854h pmid: 29675168 |
| [30] |
Huang, B.; He, Y.; Levin, M. D.; Coelho, J. A. S.; Bergman, R. G.; Toste, F. D. Adv. Synth. Catal. 2020, 362(2), 295.
doi: 10.1002/adsc.v362.2 |
| [31] |
Niu, S.; Zhang, H.; Xu, W.; Bagdi, P. R.; Zhang, G.; Liu, J.; Yang, S.; Fang, X. Nat. Commun. 2021, 12(1), 3735.
doi: 10.1038/s41467-021-23990-4 |
| [32] |
Desrues, T.; Liu, X.; Pons, J.-M.; Monnier, V.; Amalian, J.-A.; Charles, L.; Quintard, A.; Bressy, C. Org. Lett. 2021, 23(11), 4332.
doi: 10.1021/acs.orglett.1c01261 |
| [33] |
Tang, M.; Gu, H.; He, S.; Rajkumar, S.; Yang, X. Angew. Chem. Int. Ed. 2021, 60(39), 21334.
doi: 10.1002/anie.v60.39 |
| [34] |
Xie, S.; Gao, X.; Zhou, F.; Wu, H.; Zhou, J. Chin. Chem. Lett. 2020, 31(2), 324.
doi: 10.1016/j.cclet.2019.05.060 |
| [35] |
Liao, K.; Gong, Y.; Zhu, R.-Y.; Wang, C.; Zhou, F.; Zhou, J. Angew. Chem. Int. Ed. 2021, 60(15), 8488.
doi: 10.1002/anie.v60.15 |
| [36] |
Tosaki, S.-y.; Hara, K.; Gnanadesikan, V.; Morimoto, H.; Harada, S.; Sugita, M.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2006, 128(36), 11776.
doi: 10.1021/ja064858l |
| [37] |
Hara, K.; Tosaki, S.-y.; Gnanadesikan, V.; Morimoto, H.; Harada, S.; Sugita, M.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Tetrahedron 2009, 65(26), 5030.
doi: 10.1016/j.tet.2009.02.031 |
| [38] |
Shintani, R.; Takatsu, K.; Hayashi, T. Org. Lett. 2008, 10(6), 1191.
doi: 10.1021/ol800120p pmid: 18303902 |
| [39] |
Zhang, W.; Ma, S. Chem. Commun. 2018, 54(47), 6064.
doi: 10.1039/C8CC01949E |
| [40] |
Zheng, W.-F.; Zhang, W.; Huang, C.; Wu, P.; Qian, H.; Wang, L.; Guo, Y.-L.; Ma, S. Nat. Catal. 2019, 2(11), 997.
doi: 10.1038/s41929-019-0346-z |
| [41] |
Wang, J.; Zhang, W.; Wu, P.; Huang, C.; Zheng, Y.; Zheng, W.-F.; Qian, H.; Ma, S. Org. Chem. Front. 2020, 7(23), 3907.
doi: 10.1039/D0QO01106A |
| [42] |
Mao, R.; Zhao, Y.; Zhu, X.; Wang, F.; Deng, W.-Q.; Li, X. Org. Lett. 2021, 23(18), 7038.
doi: 10.1021/acs.orglett.1c02398 |
| [43] |
Kühn, F.; Katsuragi, S.; Oki, Y.; Scholz, C.; Akai, S.; Gröger, H. Chem. Commun. 2020, 56(19), 2885.
doi: 10.1039/C9CC09103C |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [4] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [5] | 胡慧娟, 闫巧丽, 卢晓刚, 杨启帆, 裴承新, 王红梅, 高润利. 猪胰脂肪酶催化外消旋P-手性α-羟基磷酸酯类化合物的动力学拆分[J]. 有机化学, 2023, 43(8): 2815-2825. |
| [6] | 褚杨杨, 韩召斌, 丁奎岭. 动力学拆分在过渡金属催化的不对称(转移)氢化中的应用研究[J]. 有机化学, 2023, 43(6): 1934-1951. |
| [7] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [8] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [9] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [10] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [11] | 陈宇亮, 贺凤开, 王思云, 贾鼎成, 刘亚群, 黄毅勇. 手性磷酸催化α-全碳季碳醛的不对称烯丙基化动力学拆分[J]. 有机化学, 2023, 43(12): 4294-4302. |
| [12] | 赵佳怡, 葛怡聪, 何川. 不对称催化Si—H/X—H脱氢偶联构筑硅中心手性[J]. 有机化学, 2023, 43(10): 3352-3366. |
| [13] | 曾燕, 叶飞. 不对称催化构建硅立体中心化合物的新反应体系研究进展[J]. 有机化学, 2023, 43(10): 3388-3413. |
| [14] | 代增进, 张绪穆, 殷勤. 铵盐为胺源的不对称还原胺化反应研究进展[J]. 有机化学, 2022, 42(8): 2261-2274. |
| [15] | 李晖, 殷亮. 铜催化的直接型插烯反应研究进展[J]. 有机化学, 2022, 42(6): 1573-1585. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||