有机化学 ›› 2022, Vol. 42 ›› Issue (5): 1501-1508.DOI: 10.6023/cjoc202201006 上一篇 下一篇
研究论文
张智鑫a, 翟彤仪a, 朱伯汉b, 钱鹏程b,*( ), 叶龙武a,c,*(
), 叶龙武a,c,*( )
)
收稿日期:2022-01-05
修回日期:2022-01-24
发布日期:2022-01-27
通讯作者:
钱鹏程, 叶龙武
作者简介:基金资助:
Zhixin Zhanga, Tongyi Zhaia, Bohan Zhub, Pengcheng Qianb( ), Longwu Yea,c(
), Longwu Yea,c( )
)
Received:2022-01-05
Revised:2022-01-24
Published:2022-01-27
Contact:
Pengcheng Qian, Longwu Ye
About author:Supported by:文章分享
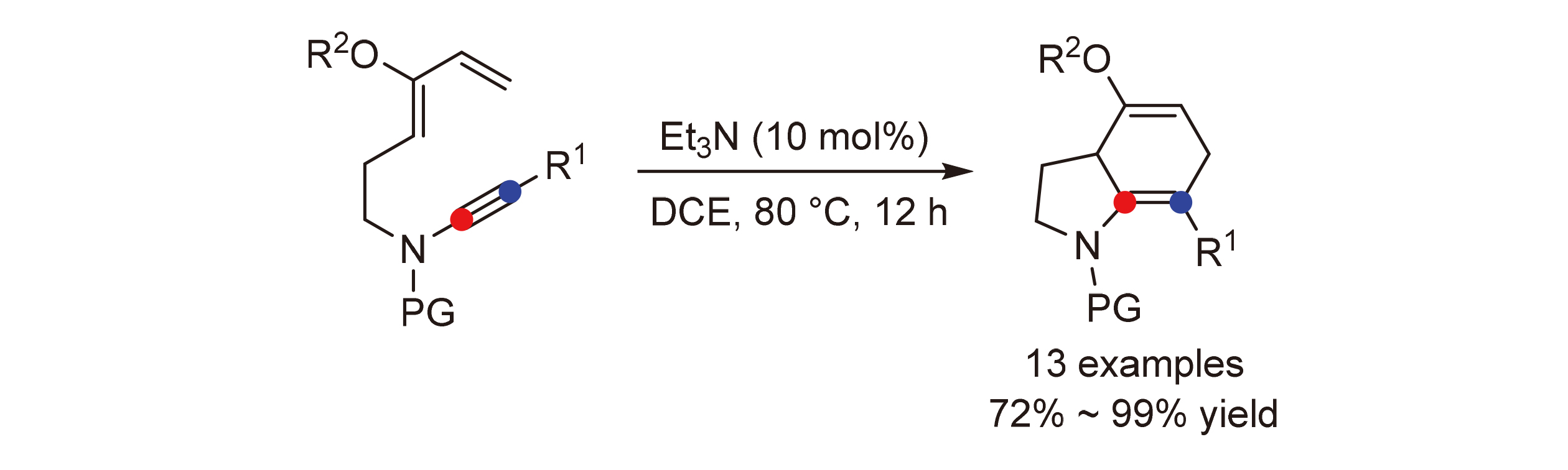
报道了无金属催化4-硅氧基-3,5-二烯炔酰胺的分子内[4+2]环化反应合成四氢吲哚衍生物的方法. 在催化量三乙胺存在下, 通过炔酰胺与硅氧基二烯的分子内Diels-Alder反应, 可方便得到六元环环化产物. 该反应条件温和、无需过渡金属催化剂、底物普适性广, 为含有4-羟基氢化吲哚骨架的天然产物和活性分子的合成提供了一条简洁和高效的途径.
张智鑫, 翟彤仪, 朱伯汉, 钱鹏程, 叶龙武. 无金属催化炔酰胺分子内[4+2]环化反应合成四氢吲哚衍生物[J]. 有机化学, 2022, 42(5): 1501-1508.
Zhixin Zhang, Tongyi Zhai, Bohan Zhu, Pengcheng Qian, Longwu Ye. Synthesis of Tetrahydroindole Derivatives via Metal-Free Intramolecular [4+2] Annulation of Ynamides[J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1501-1508.
| Entry | Catalyst | Conditions | Yieldb/% | |
|---|---|---|---|---|
| 2a | 2aa | |||
| 1 | — | DCE, 80 ℃, 12 h | <5 | <5 |
| 2 | Cu(CH3CN)4PF6 | DCE, r.t., 2 h | <5 | 20 |
| 3 | Cu(OTf)2 | DCE, r.t., 2 h | <5 | 15 |
| 4 | MsOH | DCE, r.t., 0.5 h | <5 | 22 |
| 5 | HNTf2 | DCE, r.t., 0.5 h | <5 | <5 |
| 6c | TFA | DCE, 80 ℃, 12 h | <5 | <5 |
| 7 | TfOH | DCE, r.t., 0.5 h | <5 | <5 |
| 8c | Et3N | DCE, r.t., 24 h | <5 | <1 |
| 9 | Et3N | DCE, 80 ℃, 12 h | 99 | <1 |
| 10 | Pyrrolidine | DCE, 80 ℃, 12 h | 99 | <1 |
| Entry | Catalyst | Conditions | Yieldb/% | |
|---|---|---|---|---|
| 2a | 2aa | |||
| 1 | — | DCE, 80 ℃, 12 h | <5 | <5 |
| 2 | Cu(CH3CN)4PF6 | DCE, r.t., 2 h | <5 | 20 |
| 3 | Cu(OTf)2 | DCE, r.t., 2 h | <5 | 15 |
| 4 | MsOH | DCE, r.t., 0.5 h | <5 | 22 |
| 5 | HNTf2 | DCE, r.t., 0.5 h | <5 | <5 |
| 6c | TFA | DCE, 80 ℃, 12 h | <5 | <5 |
| 7 | TfOH | DCE, r.t., 0.5 h | <5 | <5 |
| 8c | Et3N | DCE, r.t., 24 h | <5 | <1 |
| 9 | Et3N | DCE, 80 ℃, 12 h | 99 | <1 |
| 10 | Pyrrolidine | DCE, 80 ℃, 12 h | 99 | <1 |
| Entry | Substrate | Product | Yield/% | Entry | Substrate | Product | Yield/% |
|---|---|---|---|---|---|---|---|
| 1 | | | 99 | 8 | | | 88 |
| 2 | | | 94 | 9 | | | 97 |
| 3 | | | 94 | 10 | | | 99 |
| 4 | | | 84 | 11 | | | 88 (1.2∶1 dr) |
| 5 | | | 88 | 12 | | | 89 |
| 6 | | | 72 | 13 | | | 99 |
| 7 | | | 86 |
| Entry | Substrate | Product | Yield/% | Entry | Substrate | Product | Yield/% |
|---|---|---|---|---|---|---|---|
| 1 | | | 99 | 8 | | | 88 |
| 2 | | | 94 | 9 | | | 97 |
| 3 | | | 94 | 10 | | | 99 |
| 4 | | | 84 | 11 | | | 88 (1.2∶1 dr) |
| 5 | | | 88 | 12 | | | 89 |
| 6 | | | 72 | 13 | | | 99 |
| 7 | | | 86 |
| [1] |
(a) Vranesic, I.; Ofner, S.; Florà, P. J.; Bilbe, G.; Bouhelal, R.; Enz, A.; Desrayaud, S.; McAllister, K.; Kuhn, R.; Gasparini, F. Bioorg. Med. Chem. 2014, 22, 5790.
doi: 10.1016/j.bmc.2014.09.033 |
|
(b) Rafferty, M. WO 2008066874, 2008.
|
|
|
(c) Ohkawa, T.; Imamura, K.; Kurosaki, T.; Kobayashi, M.; Shimizu, S. WO 2004111041, 2004.
|
|
| [2] |
For selected examples on the applications of Diels-Alder reactions in organic synthesis, see: (a) Yang, B.; Gao, S. Chem. Soc. Rev. 2018, 47, 7926.
doi: 10.1039/C8CS00274F pmid: 26614562 |
|
(b) Gregoritza, M.; Brandl, F. P. Eur. J. Pharm. Biopharm. 2015, 97, 438.
doi: 10.1016/j.ejpb.2015.06.007 pmid: 26614562 |
|
|
(c) Nawrat, C. C.; Moody, C. J. Angew. Chem., Int. Ed. 2014, 53, 2056.
doi: 10.1002/anie.201305908 pmid: 26614562 |
|
|
(d) Takao, K.; Munakata, R.; Tadano, K. Chem. Rev. 2005, 105, 4779.
doi: 10.1021/cr040632u pmid: 26614562 |
|
|
(e) Nicolaou, K. C.; Snyder, S. A.; Montagnon, T.; Vassilikogiannakis, G. Angew. Chem., Int. Ed. 2002, 41, 1668.
doi: 10.1002/1521-3773(20020517)41:10【-逻*辑*与-】lt;1668::AID-ANIE1668【-逻*辑*与-】gt;3.0.CO;2-Z pmid: 26614562 |
|
|
(f) Corey, E. J. Angew. Chem., Int. Ed. 2002, 41, 1650.
doi: 10.1002/1521-3773(20020517)41:10【-逻*辑*与-】#x00026;lt;1650::AID-ANIE1650【-逻*辑*与-】gt;3.0.CO;2-B pmid: 26614562 |
|
| [3] |
For selected examples on Diels-Alder reactions of dienes and alkynes, see: (a) Feng, W.; Jiang, D.; Kee, C.; Liu, H.; Tan, C.-H. Chem. Asian J. 2016, 11, 390.
doi: 10.1002/asia.201500246 pmid: 20112978 |
|
(b) Kang, T.; Wang, Z.; Lin, L.; Liao, Y.; Zhou, Y.; Liu, X.; Feng, X. Adv. Synth. Catal. 2015, 357, 2045.
doi: 10.1002/adsc.201500069 pmid: 20112978 |
|
|
(c) Clary, K. N.; Parvez, M.; Back, T. G. J. Org. Chem. 2010, 75, 3751.
doi: 10.1021/jo1005087 pmid: 20112978 |
|
|
(d) Fernández de la Pradilla, R.; Tortosa, M.; Castellanos, E.; Viso, A.; Baile, R. J. Org. Chem. 2010, 75, 1517.
doi: 10.1021/jo9024489 pmid: 20112978 |
|
|
(e) Hattori, G.; Miyake, Y.; Nishibayashi, Y. ChemCatChem 2010, 2, 155.
doi: 10.1002/cctc.200900264 pmid: 20112978 |
|
|
(f) Fürstner, A.; Stimson, C. C. Angew. Chem., Int. Ed. 2007, 46, 8845.
doi: 10.1002/anie.200703321 pmid: 20112978 |
|
|
(g) Dowd, P.; Zhang, W. J. Am. Chem. Soc. 1991, 113, 9875.
doi: 10.1021/ja00026a037 pmid: 20112978 |
|
|
(h) Pindur, R.; Erfanian-Abdoust, H. Liebigs Ann. Chem. 1990, 771.
pmid: 20112978 |
|
|
(i) Birtwistlea, D. H.; Browna, J. M.; Foxton, M. W. Tetrahedron 1988, 44, 7309.
doi: 10.1016/S0040-4020(01)86103-2 pmid: 20112978 |
|
| [4] |
(a) Kim, S. M.; Parka, J. H.; Chung, Y. K. Chem. Commun. 2011, 47, 6719.
doi: 10.1039/c1cc11127b |
|
(b) Kusama, H.; Karibe, Y.; Onizawa, Y.; Iwasawa, N. Angew. Chem., Int. Ed. 2010, 49, 4269.
doi: 10.1002/anie.201001061 |
|
| [5] |
(a) Shen, M.-H.; Liang, X.-C.; Li, C.; Wu, H.; Qu, H.-Y.; Wang, F.-M.; Xu, H.-D. Tetrahedron Lett. 2019, 60, 1025.
doi: 10.1016/j.tetlet.2019.03.018 |
|
(b) Shintani, R.; Sannohe, Y.; Tsuji, T.; Hayashi, T. Angew. Chem., Int. Ed. 2007, 46, 7277.
doi: 10.1002/anie.200702586 |
|
|
(c) Lee, S. I.; Park, S. Y.; Park, J. H.; Jung, I. G.; Choi, S. Y.; Chung, Y. K.; Lee, B. Y. J. Org. Chem. 2006, 71, 91.
doi: 10.1021/jo051685u |
|
|
(d) Yoo, W.-J.; Allen, A.; Villeneuve, K.; Tam, W. Org. Lett. 2005, 7, 5853.
doi: 10.1021/ol052412o |
|
|
(e) Motoda, D.; Kinoshita, H.; Shinokubo, H.; Oshima, K. Angew. Chem., Int. Ed. 2004, 43, 1860.
doi: 10.1002/anie.200353123 |
|
|
(f) Witulski, B.; Lumtscher, J.; Bergsträßer, U. Synlett 2003, 708.
|
|
|
(g) Paik, S.-J.; Son, S. U.; Chung, Y. K. Org. Lett. 1999, 1, 2045.
doi: 10.1021/ol990169l |
|
| [6] |
Shibata, T.; Takasaku, K.; Takesue, Y.; Hirata, N.; Takagi, K. Synlett 2002, 1681.
|
| [7] |
(a) Wang, B.; Cao, P.; Zhang, X. Tetrahedron Lett. 2000, 41, 8041.
doi: 10.1016/S0040-4039(00)01432-5 |
|
(b) Wender, P. A.; Smith, T. E. Tetrahedron 1998, 54, 1255.
doi: 10.1016/S0040-4020(97)10223-X |
|
|
(c) Wender, P. A.; Jenkins, T. E. J. Am. Chem. Soc. 1989, 111, 6432.
doi: 10.1021/ja00198a071 |
|
| [8] |
For selected reviews on ynamide chemistry, see: (a) Zhao, Y.; Tu, Y.; Cai, M.; Zhao, J. Chin. J. Org. Chem. 2021, 41, 85. (in Chinese)
|
|
(赵永丽, 涂永良, 蔡明中, 赵军锋, 有机化学, 2021, 41, 85.)
|
|
|
(b) Zhou, X.; Liang, Z.; Wang, X.-N. Chin. J. Org. Chem. 2021, 41, 1288. (in Chinese)
doi: 10.6023/cjoc202009025 |
|
|
(周欣悦, 梁宗显, 王晓娜, 有机化学, 2021, 41, 1288.)
doi: 10.6023/cjoc202009025 |
|
|
(c) Tan, T.-D.; Wang, Z.-S.; Qian, P.-C.; Ye, L.-W. Small Methods 2021, 5, 2000673.
doi: 10.1002/smtd.202000673 |
|
|
(d) Hu, Y.-C.; Zhao, Y.; Wan, B.; Chen, Q.-A. Chem. Soc. Rev. 2021, 50, 2582.
doi: 10.1039/D0CS00283F |
|
|
(e) Chen, Y.-B.; Qian, P.-C.; Ye, L.-W. Chem. Soc. Rev. 2020, 49, 8897.
doi: 10.1039/D0CS00474J |
|
|
(f) Lynch, C. C.; Sripada, A.; Wolf, C. Chem. Soc. Rev. 2020, 49, 8543.
doi: 10.1039/D0CS00769B |
|
|
(g) Hong, F.-L.; Ye, L.-W. Acc. Chem. Res. 2020, 53, 2003.
doi: 10.1021/acs.accounts.0c00417 |
|
|
(h) Luo, J.; Chen, G.-S.; Chen, S.-J.; Yu, J.-S.; Li, Z.-D.; Liu, Y.-L. ACS Catal. 2020, 10, 13978.
doi: 10.1021/acscatal.0c04180 |
|
|
(i) Zhou, B.; Tan, T.-D.; Zhu, X.-Q.; Shang, M.; Ye, L.-W. ACS Catal. 2019, 9, 6393.
doi: 10.1021/acscatal.9b01851 |
|
|
(j) Pan, F.; Shu, C.; Ye, L.-W. Org. Biomol. Chem. 2016, 14, 9456.
doi: 10.1039/C6OB01774F |
|
|
(k) Evano, G.; Theunissen, C.; Lecomte, M. Aldrichim. Acta 2015, 48, 59.
|
|
|
(l) Wang, X.-N.; Yeom, H.-S.; Fang, L.-C.; He, S.; Ma, Z.-X.; Kedrowski, B. L.; Hsung, R. P. Acc. Chem. Res. 2014, 47, 560.
doi: 10.1021/ar400193g |
|
| [9] |
For recent selected examples on the ynamide chemistry in organic synthesis, see: (a) Zhu, G.; Gao, W.-C.; Jiang, X. J. Am. Chem. Soc. 2021, 143, 1334.
doi: 10.1021/jacs.0c13012 |
|
(b) Ito, T.; Harada, S.; Homma, H.; Takenaka, H.; Hirose, S.; Nemoto, T. J. Am. Chem. Soc. 2021, 143, 604.
doi: 10.1021/jacs.0c10682 |
|
|
(c) Dutta, S.; Yang, S.; Vanjari, R.; Mallick, R. K.; Gandon, V.; Sahoo, A. K. Angew. Chem., Int. Ed. 2020, 59, 10785.
doi: 10.1002/anie.201915522 |
|
|
(d) Tian, X.; Song, L.; Farshadfar, K.; Rudolph, M.; Rominger, F.; Oeser, T.; Ariafard, A.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2020, 59, 471.
doi: 10.1002/anie.201912334 |
|
|
(e) Thilmany, P.; Evano, G. Angew. Chem., Int. Ed. 2020, 59, 242.
doi: 10.1002/anie.201911722 |
|
|
(f) Yang, M.; Wang, X.; Zhao, J. ACS Catal. 2020, 10, 5230.
doi: 10.1021/acscatal.0c00523 |
|
|
(g) Prabagar, B.; Mallick, R. K.; Prasad, R.; Gandon, V.; Sahoo, A. K. Angew. Chem., Int. Ed. 2019, 58, 2365.
doi: 10.1002/anie.201813143 |
|
|
(h) Dutta, S.; Mallick, R. K.; Prasad, R.; Gandon, V.; Sahoo, A. K. Angew. Chem., Int. Ed. 2019, 58, 2289.
doi: 10.1002/anie.201811947 |
|
|
(i) Zhao, Q.; Rayo, D. F. L.; Campeau, D.; Daenen, M.; Gagosz, F. Angew. Chem., Int. Ed. 2018, 57, 13603.
doi: 10.1002/anie.201807136 |
|
|
(j) Peng, Z.-Y.; Zhang, Z.-M.; Tu, Y.-L.; Zeng, X.-Z.; Zhao, J.-F. Org. Lett. 2018, 20, 5688.
doi: 10.1021/acs.orglett.8b02409 |
|
|
(k) Zeng, L.; Huang, B.; Shen, Y.; Cui, S. Org. Lett. 2018, 20, 3460.
doi: 10.1021/acs.orglett.8b01159 |
|
|
(l) Chen, R.; Zeng, L.; Huang, B.; Shen, Y.; Cui, S. Org. Lett. 2018, 20, 3377.
doi: 10.1021/acs.orglett.8b01302 |
|
|
(m) Sahani, R. L.; Liu, R.-S. Angew. Chem., Int. Ed. 2017, 56, 12736.
doi: 10.1002/anie.201707423 |
|
|
(n) Huang, B.; Zeng, L.; Shen, Y.; Cui, S. Angew. Chem., Int. Ed. 2017, 56, 4565.
doi: 10.1002/anie.201700840 |
|
|
(o) Sahani, R. L.; Liu, R.-S. Angew. Chem., Int. Ed. 2017, 56, 1026.
doi: 10.1002/anie.201610665 |
|
|
(p) Liao, Y.; Lu, Q.; Chen, G.; Yu, Y.; Li, C.; Huang, X. ACS Catal. 2017, 7, 7529.
doi: 10.1021/acscatal.7b02558 |
|
| [10] |
For recent selected examples on transition-metal-catalyzed reactions of ynamides developed by our group, see: (a) Hong, F.-L.; Shi, C.-Y.; Hong, P.; Zhai, T.-Y.; Zhu, X.-Q.; Lu, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2022, 61, e202115554.
|
|
(b) Zhu, X.-Q.; Hong, P.; Zheng, Y.-X.; Zhen, Y.-Y.; Hong, F.-L.; Lu, X.; Ye, L.-W. Chem. Sci. 2021, 12, 9466.
doi: 10.1039/D1SC02773E |
|
|
(c) Zhu, B.-H.; Zheng, Y.-X.; Kang, W.; Deng, C.; Zhou, J.-M.; Ye, L.-W. Sci. China Chem. 2021, 64, 1985.
doi: 10.1007/s11426-021-1069-7 |
|
|
(d) Liu, X.-T.; Liu, X.; Ye, L.-W. Chin. J. Org. Chem. 2021, 41, 1207. (in Chinese)
doi: 10.6023/cjoc202009020 |
|
|
(刘晓涛, 刘鑫, 叶龙武, 有机化学, 2021, 41, 1207.)
doi: 10.6023/cjoc202009020 |
|
|
(e) Hong, F.-L.; Chen, Y.-B.; Ye, S.-H.; Zhu, G.-Y.; Zhu, X.-Q.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142, 7618.
doi: 10.1021/jacs.0c01918 |
|
|
(f) Liu, X.; Wang, Z.-S.; Zhai, T.-Y.; Luo, C.; Zhang, Y.-P.; Chen, Y.-B.; Deng, C.; Liu, R.-S.; Ye, L.-W. Angew. Chem., Int. Ed. 2020, 59, 17984.
doi: 10.1002/anie.202007206 |
|
|
(g) Huang, E.-H.; Zhang, Z.-X.; Ye, S.-H.; Chen, Y.-B.; Luo, W.-F.; Qian, P.-C.; Ye, L.-W. Chin. J. Chem. 2020, 38, 1086.
doi: 10.1002/cjoc.202000218 |
|
|
(h) Li, H.-H.; Ye, S.-H.; Chen, Y.-B.; Luo, W.-F.; Qian, P.-C.; Ye, L.-W. Chin. J. Chem. 2020, 38, 263.
doi: 10.1002/cjoc.201900478 |
|
|
(i) Hong, F.-L.; Wang, Z.-S.; Wei, D.-D.; Zhai, T.-Y.; Deng, G.-C.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2019, 141, 16961.
doi: 10.1021/jacs.9b09303 |
|
|
(j) Zheng, R.-H.; Guo, H.-C.; Yang, M.-Y.; Liu, M.-Q.; Ye, L.-W. Chin. J. Org. Chem. 2019, 39, 1672. (in Chinese)
doi: 10.6023/cjoc201903054 |
|
|
(郑人华, 郭海昌, 阳明洋, 刘梦琪, 叶龙武, 有机化学, 2019, 39, 1672.)
doi: 10.6023/cjoc201903054 |
|
|
(k) Zhu, J.; Ren, X.; Tang, F.; Pan, F.; Ye, L.-W. Chin. J. Org. Chem. 2019, 39, 1102. (in Chinese)
doi: 10.6023/cjoc201811007 |
|
|
(朱建荣, 任小娟, 唐飞宇, 潘飞, 叶龙武, 有机化学, 2019, 39, 1102.)
doi: 10.6023/cjoc201811007 |
|
|
(l) Shen, W.-B.; Xiao, X.-Y.; Sun, Q.; Zhou, B.; Zhu, X.-Q.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56, 605.
doi: 10.1002/anie.201610042 |
|
|
(m) Shen, W.-B.; Sun, Q.; Li, L.; Liu, X.; Zhou, B.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Nat. Commun. 2017, 8, 1748.
doi: 10.1038/s41467-017-01853-1 |
|
|
(n) Zhou, B.; Li, B.; Zhu, X.-Q.; Yan, J.-Z.; Guo, Y.-L.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56, 4015.
doi: 10.1002/anie.201700596 |
|
| [11] |
For recent selected examples on transition-metal-free reactions of ynamides developed by our group, see: Zhang, Y.-Q.; Chen, Y.-B.; Liu, J.-R.; Wu, S.-Q.; Fan, X.-Y.; Zhang, Z.-X.; Hong, X.; Ye, L.-W. Nat. Chem. 2021, 13, 1093.
doi: 10.1038/s41557-021-00778-z pmid: 31324800 |
|
(b) Chen, P.-F.; Zhou, B.; Wu, P.; Wang, B.; Ye, L.-W. Angew. Chem., Int. Ed. 2021, 60, 27164.
doi: 10.1002/anie.202113464 pmid: 31324800 |
|
|
(c) Zhang, Y.-Q.; Zhang, Y.-P.; Zheng, Y.-X.; Li, Z.-Y.; Ye, L.-W. Cell Rep. Phys. Sci. 2021, 2, 100448.
pmid: 31324800 |
|
|
(d) Xu, Y.; Sun, Q.; Tan, T.-D.; Yang, M.-Y.; Yuan, P.; Wu, S.-Q.; Lu, X.; Hong, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2019, 58, 16252.
doi: 10.1002/anie.201908495 pmid: 31324800 |
|
|
(e) Zhou, B. Zhang, Y.-Q.; Zhang, K.; Yang, M.-Y.; Chen, Y.-B.; Li, Y.; Peng, Q.; Zhu, S.-F.; Zhou, Q.-L.; Ye, L.-W. Nat. Commun. 2019, 10, 3234.
doi: 10.1038/s41467-019-11245-2 pmid: 31324800 |
|
|
(f) Li, L.; Zhu, X.-Q.; Zhang, Y.-Q.; Bu, H.-Z.; Yuan, P.; Chen, J.; Su, J.; Deng, X.; Ye, L.-W. Chem. Sci. 2019, 10, 3123.
doi: 10.1039/C9SC00079H pmid: 31324800 |
|
|
(g) Zhang, Y.-Q.; Zhu, X.-Q.; Xu, Y.; Bu, H.-Z.; Wang, J.-L.; Zhai, T.-Y.; Zhou, J.-M.; Ye, L.-W. Green Chem. 2019, 21, 3023.
doi: 10.1039/C9GC01030K pmid: 31324800 |
|
|
(h) Zhu, B.-H.; Wang, C.-M.; Su, H.-Y.; Ye, L.-W. Chin. J. Chem. 2019, 37, 58.
doi: 10.1002/cjoc.201800437 pmid: 31324800 |
| [1] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [2] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [3] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [4] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [5] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [6] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [7] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [8] | 纪健, 刘进华, 管丛, 陈绪文, 赵芸, 刘顺英. 原位生成的磺酸催化N-磺酰基-1,2,3-三氮唑与醇偶联高区域选择性合成N2-取代1,2,3-三氮唑[J]. 有机化学, 2023, 43(3): 1168-1176. |
| [9] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [10] | 马彪, 章淼淼, 李占宇, 彭进松, 陈春霞. 无过渡金属催化的Suzuki-Type交叉偶联反应研究进展[J]. 有机化学, 2023, 43(2): 455-470. |
| [11] | 南宁, 吴双, 秦景灏, 李金恒. 基于硅烷化启动的环化反应研究进展[J]. 有机化学, 2023, 43(10): 3414-3453. |
| [12] | 桑田, 贾帆, 何静, 李春天, 刘岩, 刘平. I2催化β-酮腈与1H-吡唑-5-胺的环化反应[J]. 有机化学, 2023, 43(1): 195-201. |
| [13] | 刘东汉, 鲁席杭, 柴张梦洁, 杨浩琦, 孙瑜琳, 余富朝. 构建2H-吡咯-2-酮骨架的研究进展[J]. 有机化学, 2023, 43(1): 57-73. |
| [14] | 王川川, 马志伟, 侯学会, 杨龙华, 陈亚静. N-Ts氰胺在有机合成中的研究与应用[J]. 有机化学, 2023, 43(1): 74-93. |
| [15] | 覃小婷, 邹宁, 农彩梅, 莫冬亮. 九元氮杂环化合物合成最新研究进展[J]. 有机化学, 2023, 43(1): 130-155. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||