有机化学 ›› 2023, Vol. 43 ›› Issue (6): 1934-1951.DOI: 10.6023/cjoc202303001 上一篇 下一篇
综述与进展
收稿日期:2023-03-01
修回日期:2023-04-08
发布日期:2023-04-26
基金资助:
Yangyang Chua,b, Zhaobin Hanb,*( ), Kuiling Dingb,c,d
), Kuiling Dingb,c,d
Received:2023-03-01
Revised:2023-04-08
Published:2023-04-26
Contact:
E-mail: Supported by:文章分享

动力学拆分是一个经典的从外消旋化合物获得光学活性物质的策略, 而过渡金属催化的不对称(转移)氢化是高效和高原子经济性地合成手性化合物的方法. 将动力学拆分与不对称(转移)氢化相结合, 基于已开发的性能优良的过渡金属催化剂, 化学家们发展了结构多样的带有不同类型手性中心的不饱和化合物的动力学拆分-不对称(转移)氢化反应, 并将其应用于多类手性药物和天然产物的不对称合成中. 根据待氢化的不饱和键类型进行分类, 综述了该领域所取得的成果, 并对后续的发展进行了展望.
褚杨杨, 韩召斌, 丁奎岭. 动力学拆分在过渡金属催化的不对称(转移)氢化中的应用研究[J]. 有机化学, 2023, 43(6): 1934-1951.
Yangyang Chu, Zhaobin Han, Kuiling Ding. Progresses in the Application of Kinetic Resolution in Transition Metal Catalyzed Asymmetric (Transfer) Hydrogenation[J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1934-1951.



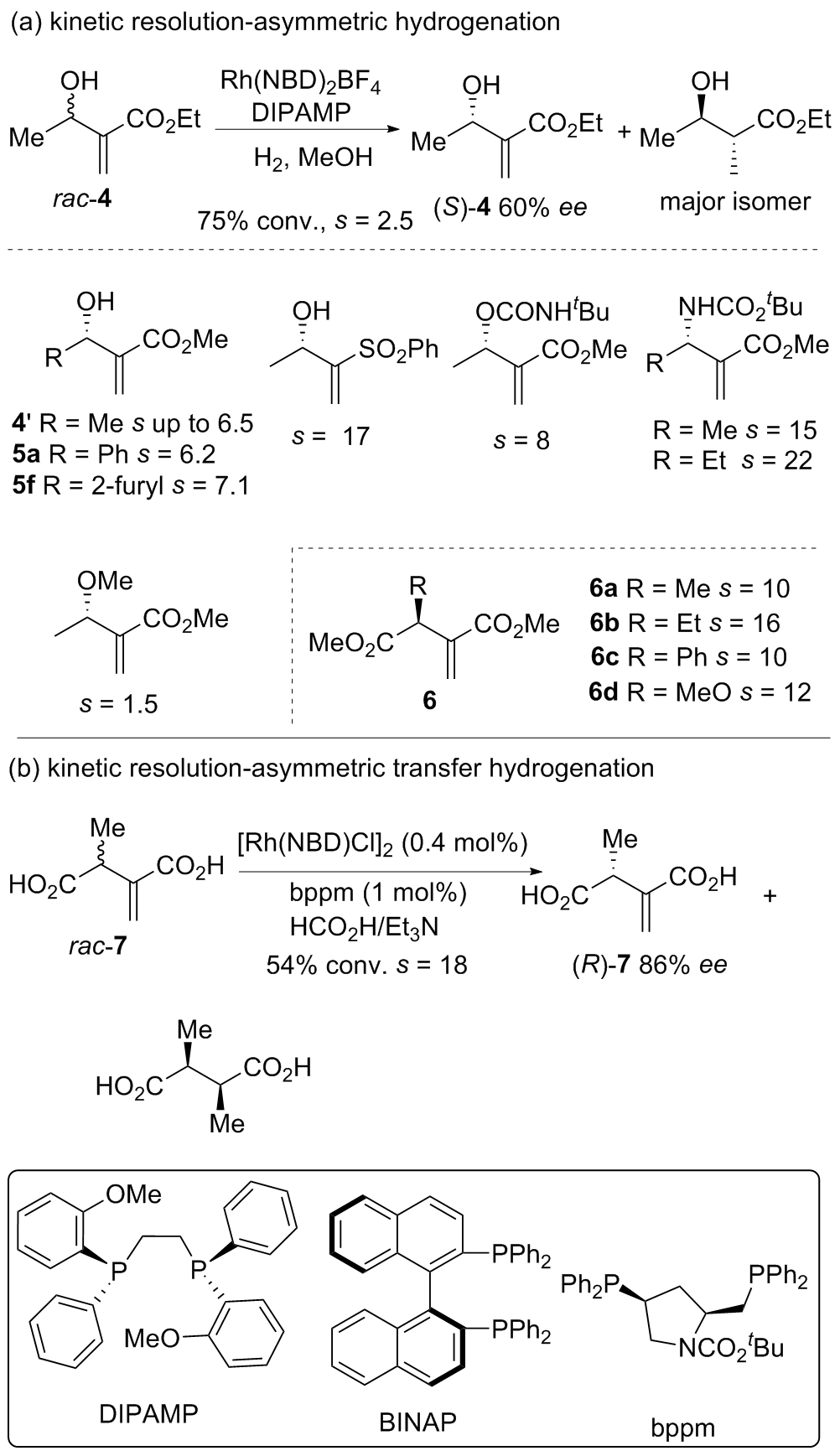

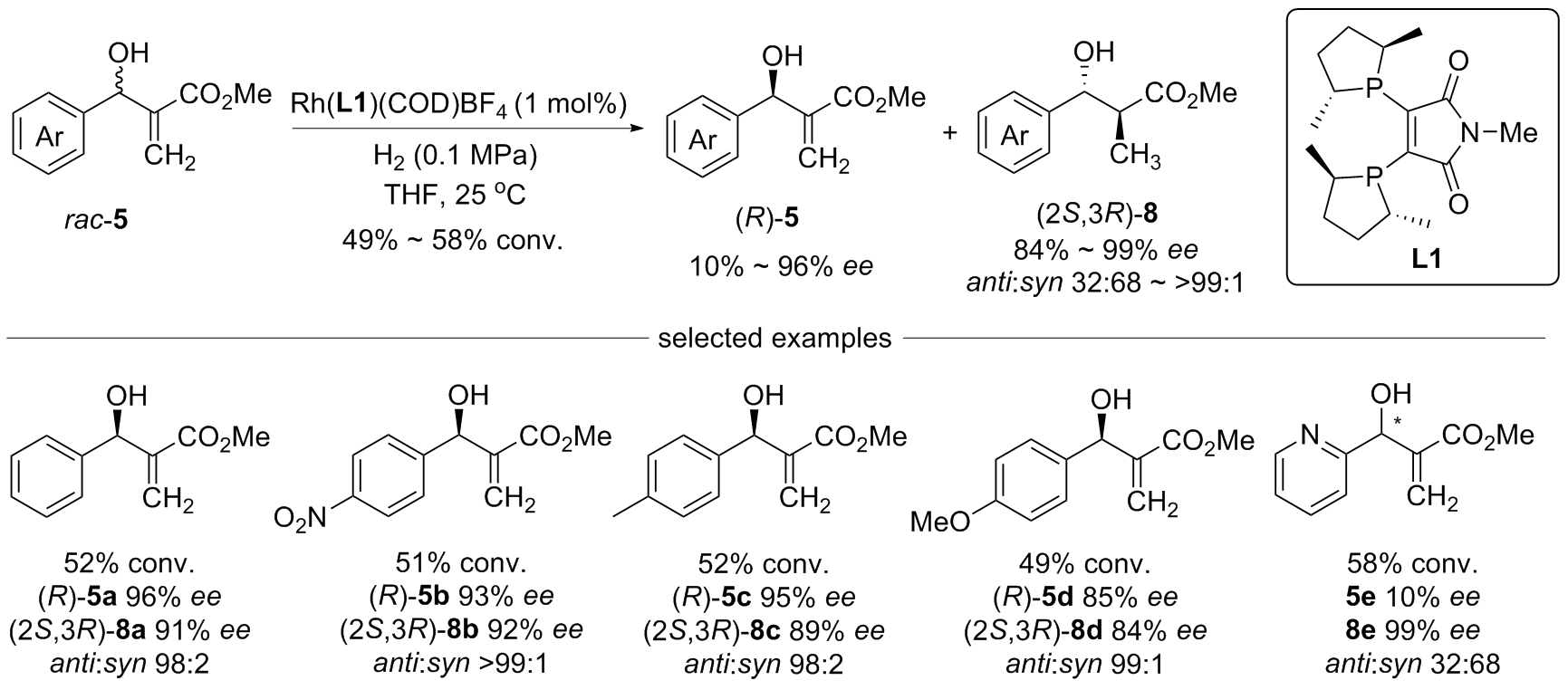
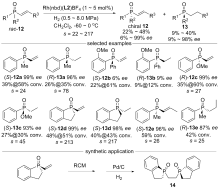
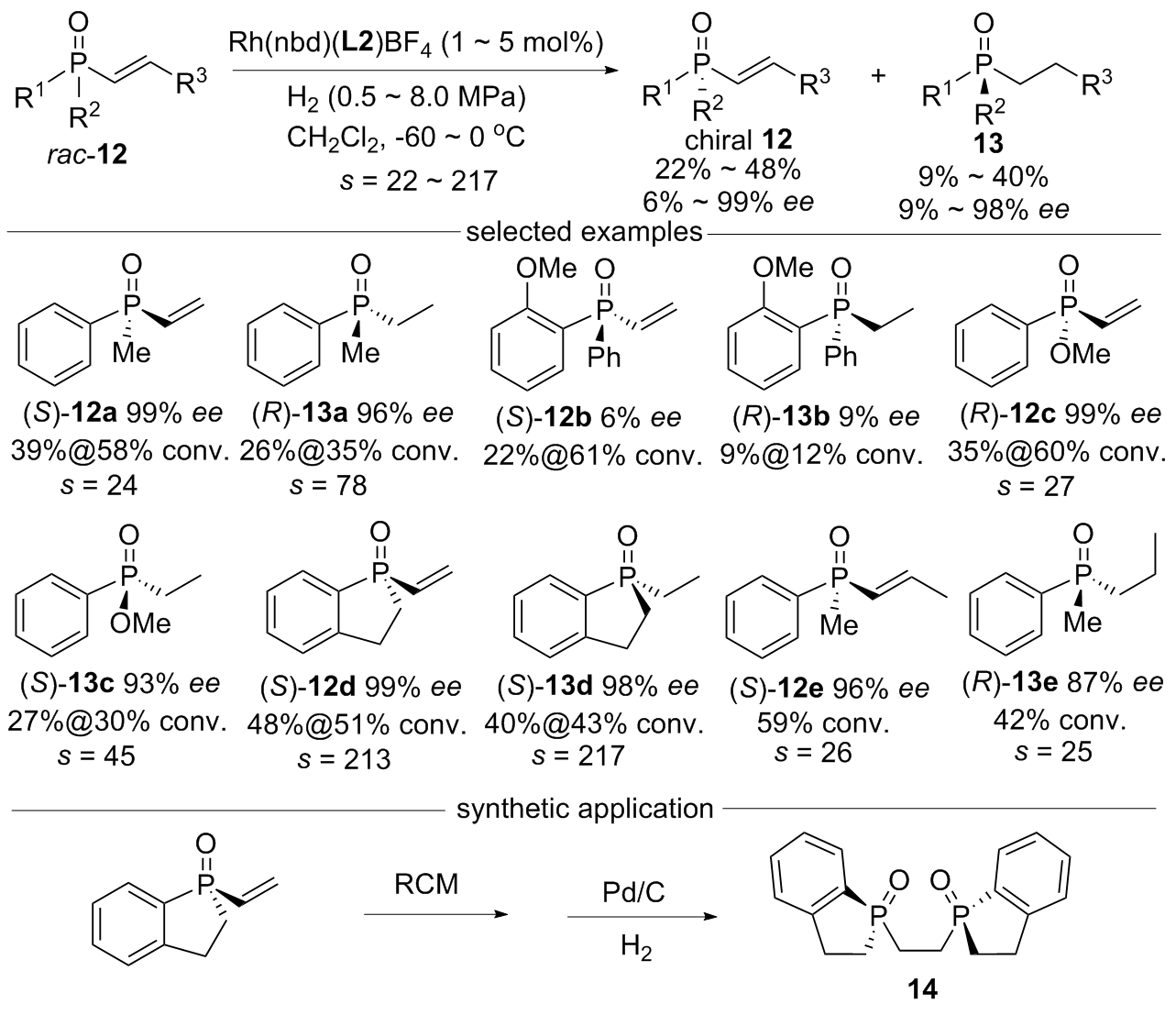





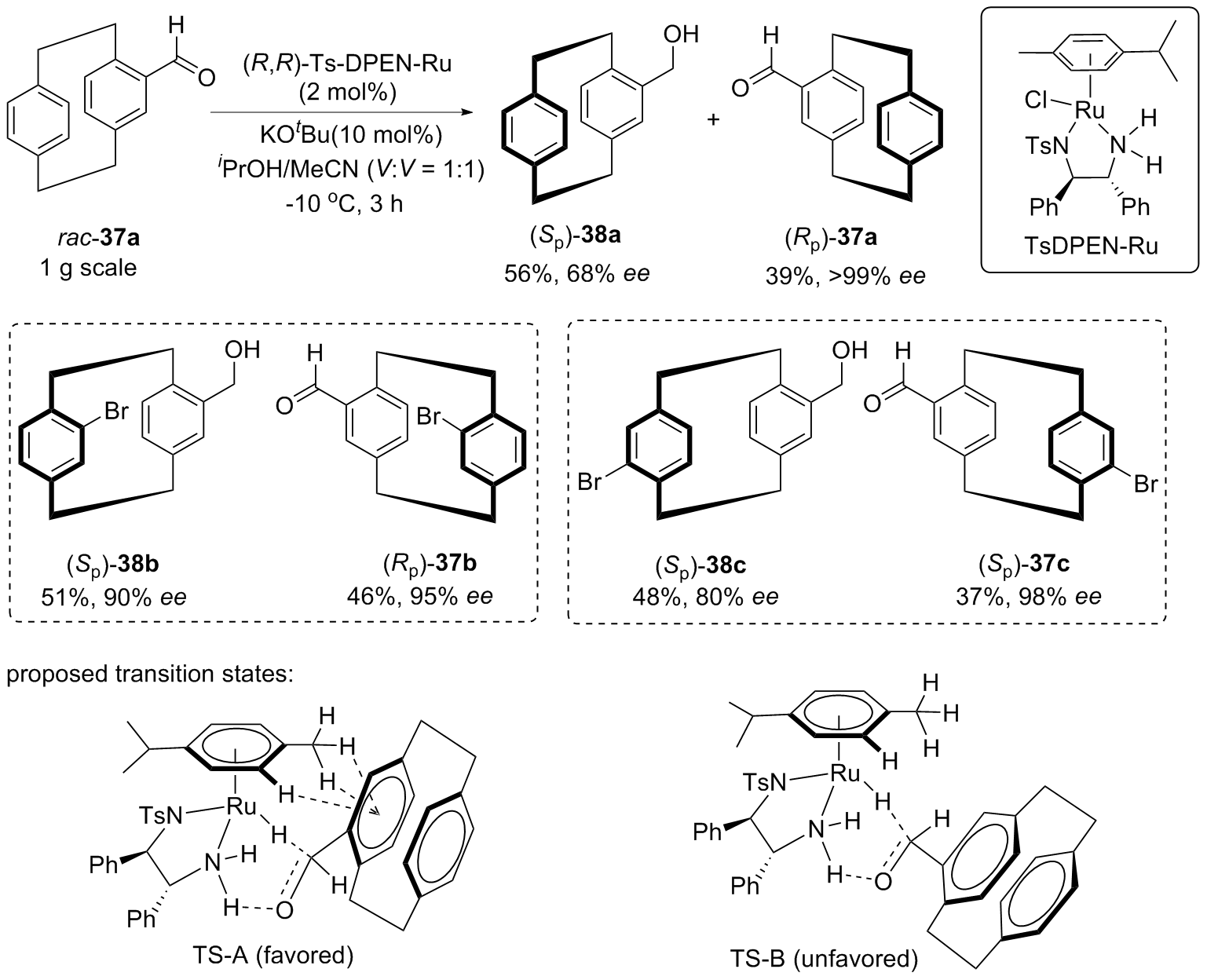
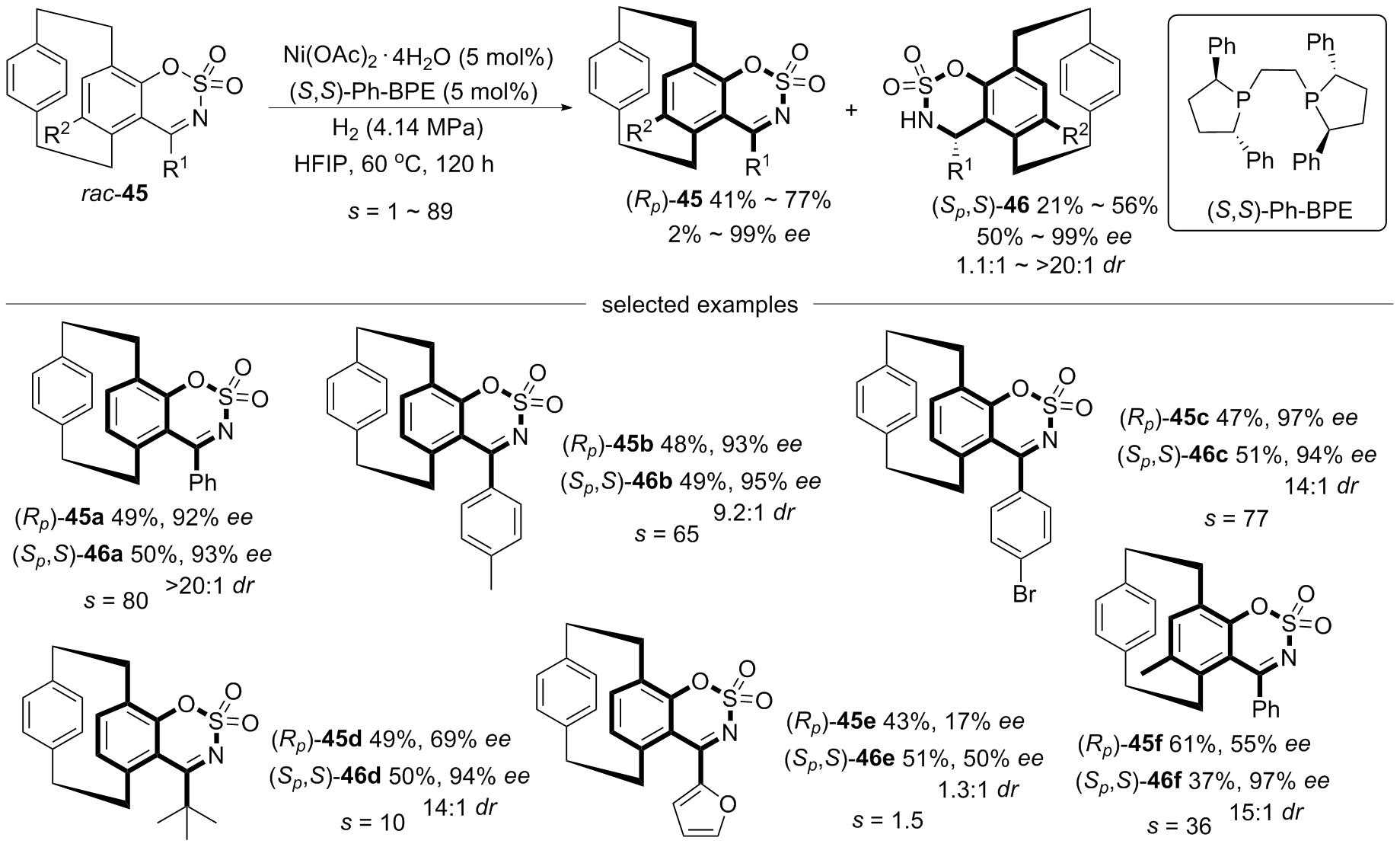
| [1] |
Ojima, I. Catalytic Asymmetric Synthesis, 3rd ed., Wiley, Hoboken, 2010.
|
| [2] |
(a) Moss, G. P. Pure Appl. Chem. 1996, 68, 2193.
doi: 10.1351/pac199668122193 |
|
(b) Keith, J. M.; Larrow, J. F.; Jacobsen, E. N. Adv. Synth. Catal. 2001, 343, 5.
doi: 10.1002/(ISSN)1615-4169 |
|
|
(c) Vedejs, E.; Jure, M. Angew. Chem., nt. Ed. 2005, 44, 3974.
|
|
|
(d) Pellissier, H. Adv. Synth. Catal. 2011, 353, 1613.
doi: 10.1002/adsc.201100111 |
|
|
(e) Su, N.; Zhang, F.; Gong, Y. Chin. J. Org. Chem. 2007, 27, 1345. (in Chinese)
doi: 10.1002/cjoc.v27:7 |
|
|
(苏宁, 张方林, 龚跃法, 有机化学, 2007, 27, 1345.)
|
|
| [3] |
Kagan, H. B.; Fiaud, J. C. Top. Stereochem. 1988, 18, 249.
|
| [4] |
Bhat, V.; Welin, E. R.; Guo, X.; Stoltz, B. M. Chem. Rev. 2017, 117, 4528.
doi: 10.1021/acs.chemrev.6b00731 |
| [5] |
Vedejs, E.; Chen, X. J. Am. Chem. Soc. 1997, 119, 2584.
doi: 10.1021/ja963666v |
| [6] |
(a) de Vries, J. G.; Elsevier, C. J. Handbook of Homogeneous Hydrogenation, Vol. 1-3, Wiley, Weinheim, 2010.
|
|
(b) Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029.
doi: 10.1021/cr020049i |
|
|
(c) Xie, J.-H.; Zhu, S.-F.; Zhou, Q.-L. Chem. Rev. 2011, 111, 1713.
doi: 10.1021/cr100218m |
|
|
(d) Wang, D.-S.; Chen, Q.-A.; Lu, S.-M.; Zhou, Y.-G. Chem. Rev. 2012, 112, 2557.
doi: 10.1021/cr200328h |
|
|
(e) Zhang, Z.; Butt, N. A.; Zhang, W. Chem. Rev. 2016, 116, 14769.
doi: 10.1021/acs.chemrev.6b00564 |
|
|
(f) Zhang, Z.; Butt, N. A.; Zhou, M.; Liu, D.; Zhang, W. Chin. J. Chem. 2018, 36, 443,
doi: 10.1002/cjoc.v36.5 |
|
|
(g) Mu, B.-S.; Zhang, Z.-H.; Wu, W.-B.; Yu, J.-S.; Zhou, J. Acta Chim. Sinica 2021, 79, 685. (in Chinese)
doi: 10.6023/A21040131 |
|
|
(穆博帅, 张志豪, 武文彪, 余金生, 周剑, 化学学报, 2021, 79, 685.)
doi: 10.6023/A21040131 |
|
|
(h) Shang, Y.; Xiao, J.; Wang, Y.; Peng, Y. Acta Chim. Sinica 2021, 79, 1303. (in Chinese)
doi: 10.6023/A21070345 |
|
|
(尚阳, 肖检, 王雅雯, 彭羽, 化学学报, 2021, 79, 1303.)
doi: 10.6023/A21070345 |
|
| [7] |
El-Baba, S.; Poulin, J.-C.; Kagan, H. B. Tetrahedron 1984, 40, 4275.
doi: 10.1016/S0040-4020(01)98803-9 |
| [8] |
Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659.
doi: 10.1021/cr300192h |
| [9] |
Brown, J. M.; Cutting, I. J. Chem. Soc., hem. Commun. 1985, 578.
|
| [10] |
Ando, D.; Bevan, C.; Brown, J. M.; Price, D. W. J. Chem. Soc., hem. Commun. 1992, 592.
|
| [11] |
Brown, J. M.; James, A. P.; Prior, L. M. Tetrahedron Lett. 1987, 28, 2179.
doi: 10.1016/S0040-4039(00)96075-1 |
| [12] |
(a) Brown, J. M. Angew. Chem.,Int. Ed. 1987, 26, 190.
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Brown, J. M.; Cutting, I.; James, A. P. Bull. Soc. Chim. Fr. 1988, 211.
|
|
| [13] |
Leitner, W.; Brown, J. M.; Brunner, H. J. Am. Chem. Soc. 1993, 115, 152.
doi: 10.1021/ja00054a021 |
| [14] |
Holz, J.; Schäffner, B.; Zayas, O.; Spannenberg, A.; Börner, A. Adv. Synth. Catal. 2008, 350, 2533.
doi: 10.1002/adsc.v350:16 |
| [15] |
(a) Trost, B. M.; Rao, M. Angew. Chem.,Int. Ed. 2015, 54, 5026.
doi: 10.1002/anie.v54.17 |
|
(b) Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kiełbasiński, P. Chem. Rev. 2017, 117, 4147.
doi: 10.1021/acs.chemrev.6b00517 |
|
| [16] |
(a) Bentley, R. Chem. Soc. Rev. 2005, 34, 609.
pmid: 15965542 |
|
(b) Legros, J.; Dehli, J. R.; Bolm, C. Adv. Synth. Catal. 2005, 347, 19.
doi: 10.1002/(ISSN)1615-4169 pmid: 15965542 |
|
| [17] |
(a) Mikolajczyk, M.; Drabowicz, J. Phosphrous, Sulfur Silicon Relat. Elem. 1976, 1, 301.
|
|
(b) Annunziata, R.; Borgogno, G.; Montanari, F.; Quici, S.; Cucinella, S. J. Chem. Soc., erkin Trans. 1 1981, 113.
|
|
| [18] |
Lao, J. R.; Fernández-Pérez, H.; Vidal-Ferran, A. Org. Lett. 2015, 17, 4114.
doi: 10.1021/acs.orglett.5b02139 |
| [19] |
Fernández-Pérez, H.; Lao, J. R.; Grabulosa, A.; Vidal-Ferran, A. Eur. J. Org. Chem. 2020, 2020, 4331.
doi: 10.1002/ejoc.202000592 |
| [20] |
Dutartre, M.; Bayardon, J.; Jugé, S. Chem. Soc. Rev. 2016, 45, 5771.
pmid: 27479243 |
| [21] |
Fernández-Pérez, H.; Vidal-Ferran, A. Org. Lett. 2019, 21, 7019.
doi: 10.1021/acs.orglett.9b02606 pmid: 31461296 |
| [22] |
Buffat, M. G. P. Tetrahedron 2004, 60, 1701.
doi: 10.1016/j.tet.2003.11.043 |
| [23] |
Zhou, C.-X.; Zhang, W.; You, S.-L. Angew. Chem., nt. Ed. 2012, 51, 12662.
|
| [24] |
Li, W.; Yang, H.; Li, R.; Lv, H.; Zhang, X. ACS Catal. 2020, 10, 2603.
doi: 10.1021/acscatal.9b05444 |
| [25] |
(a) Kitamura, M.; Kasahara, I.; Manabe, K.; Noyori, R.; Takaya, H. J. Org.Chem. 1998, 53, 710.
|
|
(b) Mikami, K.; Yusa, Y.; Korenaga, T. Org. Lett. 2002, 1643.
|
|
| [26] |
Wu, H.; Margarita, C.; Jongcharoenkamol, J.; Nolan, M. D.; Singh, T.; Andersson, P. G. Chem. Sci. 2021, 12, 1937.
doi: 10.1039/D0SC05276K |
| [27] |
Wiesenfeldt, M. P.; Nairoukh, Z.; Dalton, T.; Glorius, F. Angew. Chem., nt. Ed. 2019, 58, 10460.
|
| [28] |
Ding, Y.-X.; Zhu, Z.-H.; Chen, M.-W.; Yu, C.-B.; Zhou, Y.-G. Angew. Chem., nt. Ed. 2022, 61, e202205623.
|
| [29] |
Veitch, N. C.; Grayer, R. J. Nat. Prod. Rep. 2011, 28, 1626.
doi: 10.1039/c1np00044f |
| [30] |
(a) Noyori, R.; Ohkuma, T. Angew. Chem., nt. Ed. 2001, 40, 40.
|
|
(b) Xie, J.-H.; Bao, D.-H.; Zhou, Q.-L. Synthesis 2015, 47, 460.
doi: 10.1055/s-00000084 |
|
| [31] |
Lemke, M.-K.; Schwab, P.; Fischer, P.; Tischer, S.; Witt, M.; Noehringer, L.; Rogachev, V.; Jäger, A.; Kataeva, O.; Fröhlich, R.; Metz, P. Angew. Chem., nt. Ed. 2013, 52, 11651.
|
| [32] |
Keßberg, A.; Metz, P. Angew. Chem., nt. Ed. 2016, 55, 1160.
|
| [33] |
Keßberg, A.; Metz, P. Org. Lett. 2016, 18, 6500.
pmid: 27978700 |
| [34] |
He, B.; Phansavath, P.; Ratovelomanana-Vidal, V. Org. Chem. Front. 2021, 8, 2504.
doi: 10.1039/D1QO00141H |
| [35] |
Zhu, Y.; Zhou, J.; Li, J.; Xu, K.; Ye, J.; Lu, Y.; Liu, D.; Zhang, W. Org. Chem. Front. 2021, 8, 6609.
doi: 10.1039/D1QO01310F |
| [36] |
Park, S.; Lee, H.-K. RSC Adv. 2021, 11, 23161.
doi: 10.1039/D1RA04538E |
| [37] |
(a) Müller, C. E.; Schreiner, P. R. Angew. Chem., nt. Ed. 2011, 50, 6012.
|
|
(b) Sigman, M. S.; Jensen, D. R. Acc. Chem. Res. 2006, 39, 221.
doi: 10.1021/ar040243m |
|
| [38] |
(a) Yang, X.-H.; Wang, K.; Zhu, S.-F.; Xie, J.-H.; Zhou, Q.-L. J. Am. Chem. Soc. 2014, 136, 17426.
doi: 10.1021/ja510990v |
|
(b) Yang, X.-H.; Gu, X.-S.; Bin, H.-Y.; Xie, J.-H.; Zhou, Q.-L. Chin. J. Org. Chem. 2020, 40, 3963. (in Chinese)
doi: 10.6023/cjoc202007052 |
|
|
(杨小会, 顾雪松, 宾怀玉, 谢建华, 周其林, 有机化学, 2020, 40, 3963.)
doi: 10.6023/cjoc202007052 |
|
| [39] |
Dai, L.-X.; Hou, X.-L. Chiral Ferrocenes in Asymmetric Catalysis, Wiley, Weinheim, 2010.
|
| [40] |
Liu, C.-X.; Zhao, F.; Feng, Z.; Wang, Q.; Gu, Q.; You, S.-L. Nat. Synth. 2023, 2, 49.
doi: 10.1038/s44160-022-00177-3 |
| [41] |
Liu, R.; Zhou, G.; Hall, T. H.; Clarkson, G. J.; Wills, M.; Chen, W. Adv. Synth. Catal. 2015, 357, 3453.
doi: 10.1002/adsc.201500728 |
| [42] |
Delcourt, M.-L.; Turcaud, S.; Benedetti, E.; Micouin, L. Adv. Synth. Catal. 2016, 358, 1213.
doi: 10.1002/adsc.v358.8 |
| [43] |
Delcourt, M.-L.; Felder, S.; Turcaud, S.; Pollok, C. H.; Merten, C.; Benedetti, E.; Micouin, L. J. Org. Chem. 2019, 84, 5369.
doi: 10.1021/acs.joc.9b00372 |
| [44] |
Nugent, T. C. Chiral Amine Synthesis, Wiley, Weinheim, 2010.
|
| [45] |
Lensink, C.; de Vries, J. G. Tetrahedron: Asymmetry 1993, 4, 215.
|
| [46] |
Viso, A.; Lee, N. E.; Buchwald, S. L. J. Am. Chem. Soc. 1994, 116, 9373.
doi: 10.1021/ja00099a082 |
| [47] |
Yang, Z.; Chen, F.; He, Y.; Yang, N.; Fan, Q.-H. Angew. Chem., nt. Ed. 2016, 55, 13863.
|
| [48] |
Liu, C.; Wang, M.; Xu, Y.; Li, Y.; Liu, Q. Angew. Chem., nt. Ed. 2022, 61, e202202814.
|
| [49] |
Zhao, Y.; Ding, Y.-X.; Wu, B.; Zhou, Y.-G. J. Org. Chem. 2021, 86, 10788.
doi: 10.1021/acs.joc.1c01011 |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [3] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [4] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [5] | 王文芳. 过渡金属催化不对称C—H硼化反应研究进展[J]. 有机化学, 2023, 43(9): 3146-3166. |
| [6] | 高晓阳, 翟锐锐, 陈训, 王烁今. 碳酸亚乙烯酯参与C—H键活化反应的研究进展[J]. 有机化学, 2023, 43(9): 3119-3134. |
| [7] | 胡慧娟, 闫巧丽, 卢晓刚, 杨启帆, 裴承新, 王红梅, 高润利. 猪胰脂肪酶催化外消旋P-手性α-羟基磷酸酯类化合物的动力学拆分[J]. 有机化学, 2023, 43(8): 2815-2825. |
| [8] | 周章涛, 王杨, 程冰心, 叶伟平. [RuCl(p-cymene)-(S)-BINAP]Cl催化不对称合成反式-3-氨基-双环[2.2.2]辛烷-2-甲酸乙酯[J]. 有机化学, 2023, 43(8): 2961-2967. |
| [9] | 陈新强, 张敬. 伯醇的脱羟甲基反应的研究进展[J]. 有机化学, 2023, 43(8): 2711-2719. |
| [10] | 石义军, 孙馨悦, 曹晗, 别福升, 马杰, 刘哲, 丛兴顺. 室温下酯与伯硫醇的硫酯化反应[J]. 有机化学, 2023, 43(7): 2499-2505. |
| [11] | 董思凡, 李昊龙, 秦源, 范士明, 刘守信. 氨基酸作为瞬态导向基在碳氢键活化反应中的研究进展[J]. 有机化学, 2023, 43(7): 2351-2367. |
| [12] | 徐忠荣, 万结平, 刘云云. 基于热、光以及电化学过程的无过渡金属碳-氢键硫氰化和硒氰化反应[J]. 有机化学, 2023, 43(7): 2425-2446. |
| [13] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [14] | 徐光利, 许静, 徐海东, 崔香, 舒兴中. 过渡金属催化烯烃和炔烃合成1,3-共轭二烯化合物研究进展[J]. 有机化学, 2023, 43(6): 1899-1933. |
| [15] | 户晓兢, 郭斐翔, 朱润青, 周柄棋, 张涛, 房立真. 对烷氧基酚的合成及其去芳构化后的合成应用[J]. 有机化学, 2023, 43(6): 2239-2244. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||