有机化学 ›› 2023, Vol. 43 ›› Issue (10): 3526-3543.DOI: 10.6023/cjoc202306007 上一篇 下一篇
所属专题: 二氧化碳虚拟合辑; 有机硅化学专辑-2023
综述与进展
收稿日期:2023-06-11
修回日期:2023-08-07
发布日期:2023-08-30
基金资助:
Peifeng Sua, Jinyu Nia, Zhuofeng Kea,b( )
)
Received:2023-06-11
Revised:2023-08-07
Published:2023-08-30
Contact:
*E-mail: Supported by:文章分享

利用二氧化碳作为C1原料进行硅氢化转化, 是可持续性催化合成的重要方法之一. 该方法能够将二氧化碳转化为不同氧化水平的高值化学品, 例如甲酸、甲醛、甲醇和甲烷等. 此外, 胺可在特定的催化体系中与二氧化碳和硅烷多组分反应, 实现基于二氧化碳硅氢化的N—H键酰基化和烷基化等转化. 近年来, 二氧化碳硅氢化领域的相关研究取得了显著的进展. 综述了近三年来应用于二氧化碳硅氢化的主要均相催化体系的研究进展, 介绍和总结催化剂的设计和相关催化性能, 包括贵金属催化、廉价过渡金属催化、稀土金属催化、主族金属催化和无金属催化等催化体系, 并讨论和展望了目前二氧化碳硅氢化的研究现状和潜在挑战.
苏沛锋, 倪金煜, 柯卓锋. 二氧化碳硅氢化及相关转化的均相催化体系研究进展[J]. 有机化学, 2023, 43(10): 3526-3543.
Peifeng Su, Jinyu Ni, Zhuofeng Ke. Recent Advances in Homogeneous Catalytic Systems for CO2 Hydrosilylation and Related Transformations[J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3526-3543.



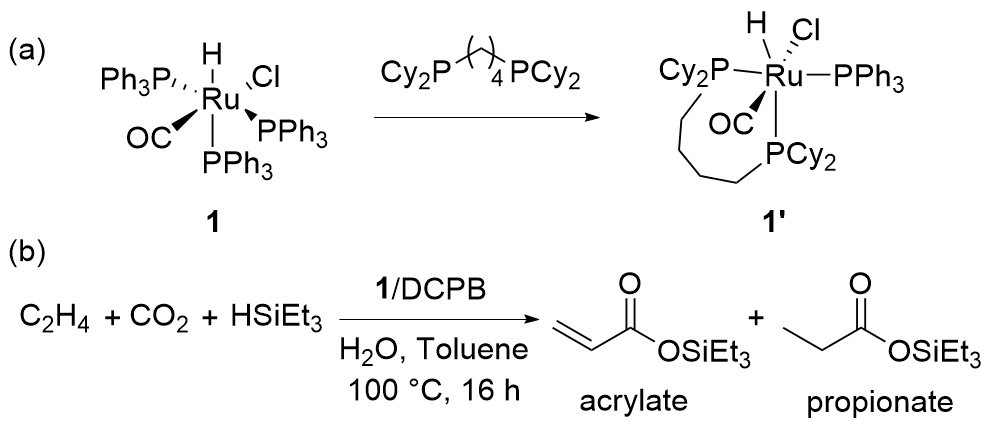

| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactant | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 1/DCPB[ | HSiEt3 | 1 | C2H4 | Toluene/H2O | 100 | 16 | Acrylate+propionate |
| 2 | 2[ | HSiMe2Ph | 0.3 | Amine | C6D6 | 50 | 14 | Silylcarbamates |
| 3 | 3/B(C6F5)3[ | HMTS, HSiMe2Ph, HSiMePh2, or HSiEt3 | 0.1 | — | C6D6 | 50 | 8~40 | Bis(silyl)acetals |
| 4 | 4[ | HSiMe(OSiMe3)2, HSiMe2Ph, or HSiMePh2 | 0.27 | — | C6D6 | 50 | 3~24 | Silylcarbonates+silylfor- mates+methoxysilanes |
| 5 | 5[ | HSiMe2Ph, HSiMePh2, or HSiEt3 | 0.3 | — | CD3CN | 75 | 2~10 | Silylformates |
| 6 | 5[ | HSiMe2Ph | 0.3 | Amine | CD3CN | 75 | 15 | Silylcarbamates |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactant | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 1/DCPB[ | HSiEt3 | 1 | C2H4 | Toluene/H2O | 100 | 16 | Acrylate+propionate |
| 2 | 2[ | HSiMe2Ph | 0.3 | Amine | C6D6 | 50 | 14 | Silylcarbamates |
| 3 | 3/B(C6F5)3[ | HMTS, HSiMe2Ph, HSiMePh2, or HSiEt3 | 0.1 | — | C6D6 | 50 | 8~40 | Bis(silyl)acetals |
| 4 | 4[ | HSiMe(OSiMe3)2, HSiMe2Ph, or HSiMePh2 | 0.27 | — | C6D6 | 50 | 3~24 | Silylcarbonates+silylfor- mates+methoxysilanes |
| 5 | 5[ | HSiMe2Ph, HSiMePh2, or HSiEt3 | 0.3 | — | CD3CN | 75 | 2~10 | Silylformates |
| 6 | 5[ | HSiMe2Ph | 0.3 | Amine | CD3CN | 75 | 15 | Silylcarbamates |


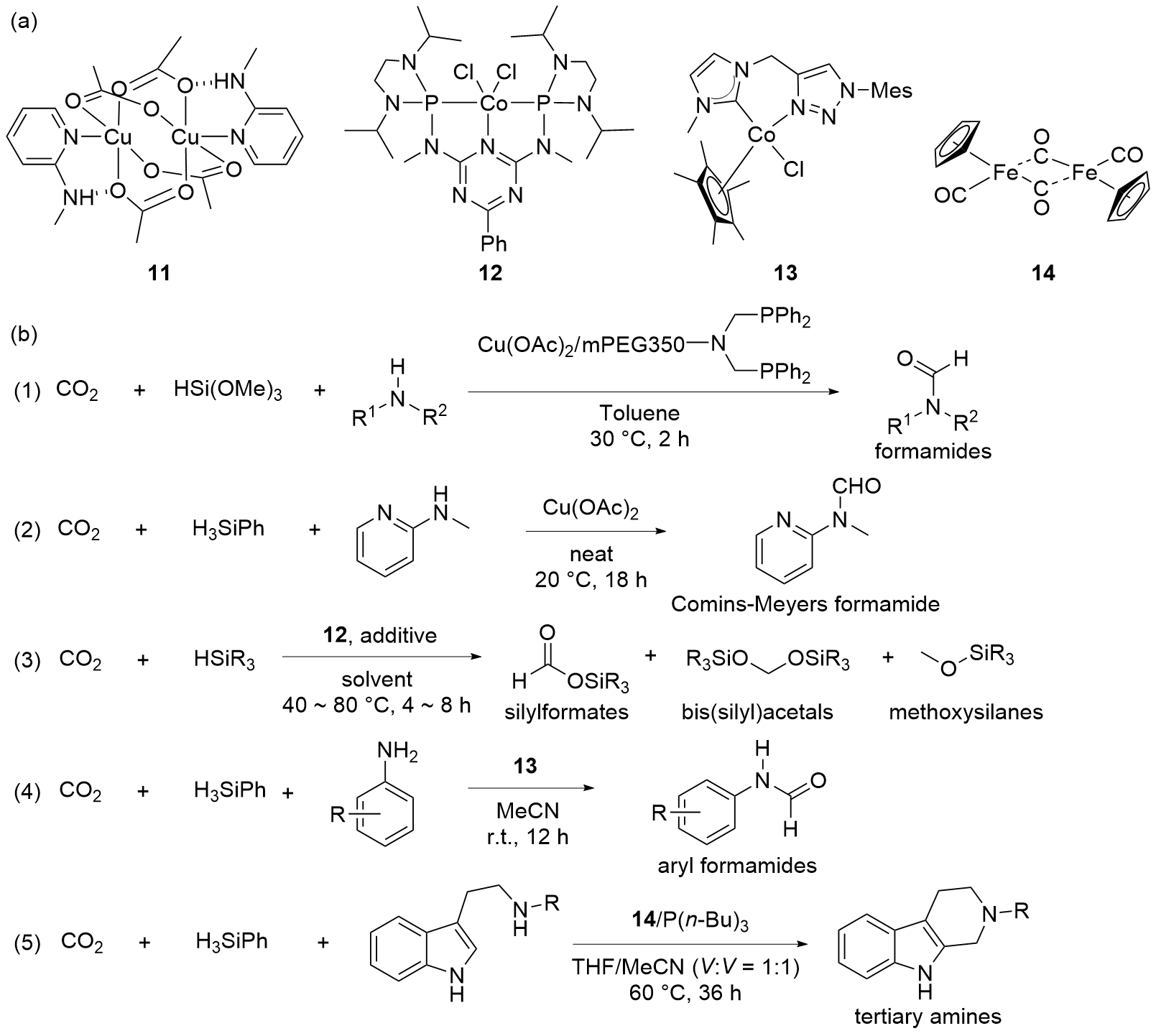
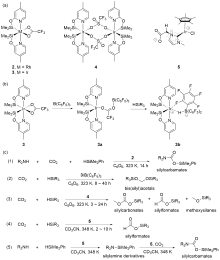
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 6[ | H3SiPh | 0.7 | — | THF | 100 | 72 | Methoxysilanes |
| 2 | Mn(CO)5Br[ | HSiEt3 | 0.4 | — | THF or THF/toluene | 50 | 1 | Silylformates+ bis(silyl)acetals |
| 3a | 7[ | H3SiPh | 0.1 | Secondary amides or lactams | MeCN | r.t. | 12 | Acyl formamides |
| 4 | 8[ | H3SiPh | 1.5 | Amines | MeCN | r.t. | 24 | Formamidesb |
| 5 | 8[ | H3SiPh | 0.1 | Amines | MeCN | r.t. | 24 | Formamides |
| 6 | 8[ | H3SiPh | 0.1 | Amines | MeCN | 100 | 15 | Tertiary amine |
| 7 | 9[ | H3SiPh | 0.1 | — | DMSO-d6 | 25 | 1~24 | Silylformates+methoxysilanes |
| 8 | 9[ | H3SiPh or H2SiPh2 | 0.1 | — | DMSO-d6 | 80 | 1~24 | Methoxysilanes |
| 9 | 10[ | H2SiMePh | 0.1 | — | C6D6 | 70 | 8 | Silanol |
| 10 | Cu(OAc)2/ mPEG-PNP[ | HSi(OMe)3 | 0.1 | Amines | Toluene | 30 | 2 | Formamides |
| 11 | Cu(OAc)2[ | H3SiPh | 0.1 | 2-(Methylamino)pyridine | Neat | 20 | 18 | Formamides |
| 12c | 12[ | H2SiPh2 | 0.1 | — | Neat | 40 | 4 | Silylformates |
| 13c | 12[ | H3SiPh | 0.1 | — | Neat | 80 | 8 | Bis(silyl)acetals |
| 14c | 12[ | H3SiPh | 0.1 | — | DMSO-d6 | 80 | 4 | Methoxysilanes |
| 15 | 13[ | H3SiPh | 0.1 | Anilines | MeCN | r.t. | 12 | Aryl formamides |
| 16 | 14/P(n-Bu)3[ | H3SiPh | 0.1 | Tryptamine derivative | THF/MeCN (V∶V=1∶1) | 60 | 36 | Tertiary amines |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 6[ | H3SiPh | 0.7 | — | THF | 100 | 72 | Methoxysilanes |
| 2 | Mn(CO)5Br[ | HSiEt3 | 0.4 | — | THF or THF/toluene | 50 | 1 | Silylformates+ bis(silyl)acetals |
| 3a | 7[ | H3SiPh | 0.1 | Secondary amides or lactams | MeCN | r.t. | 12 | Acyl formamides |
| 4 | 8[ | H3SiPh | 1.5 | Amines | MeCN | r.t. | 24 | Formamidesb |
| 5 | 8[ | H3SiPh | 0.1 | Amines | MeCN | r.t. | 24 | Formamides |
| 6 | 8[ | H3SiPh | 0.1 | Amines | MeCN | 100 | 15 | Tertiary amine |
| 7 | 9[ | H3SiPh | 0.1 | — | DMSO-d6 | 25 | 1~24 | Silylformates+methoxysilanes |
| 8 | 9[ | H3SiPh or H2SiPh2 | 0.1 | — | DMSO-d6 | 80 | 1~24 | Methoxysilanes |
| 9 | 10[ | H2SiMePh | 0.1 | — | C6D6 | 70 | 8 | Silanol |
| 10 | Cu(OAc)2/ mPEG-PNP[ | HSi(OMe)3 | 0.1 | Amines | Toluene | 30 | 2 | Formamides |
| 11 | Cu(OAc)2[ | H3SiPh | 0.1 | 2-(Methylamino)pyridine | Neat | 20 | 18 | Formamides |
| 12c | 12[ | H2SiPh2 | 0.1 | — | Neat | 40 | 4 | Silylformates |
| 13c | 12[ | H3SiPh | 0.1 | — | Neat | 80 | 8 | Bis(silyl)acetals |
| 14c | 12[ | H3SiPh | 0.1 | — | DMSO-d6 | 80 | 4 | Methoxysilanes |
| 15 | 13[ | H3SiPh | 0.1 | Anilines | MeCN | r.t. | 12 | Aryl formamides |
| 16 | 14/P(n-Bu)3[ | H3SiPh | 0.1 | Tryptamine derivative | THF/MeCN (V∶V=1∶1) | 60 | 36 | Tertiary amines |
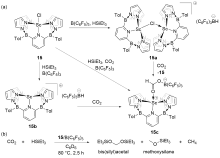
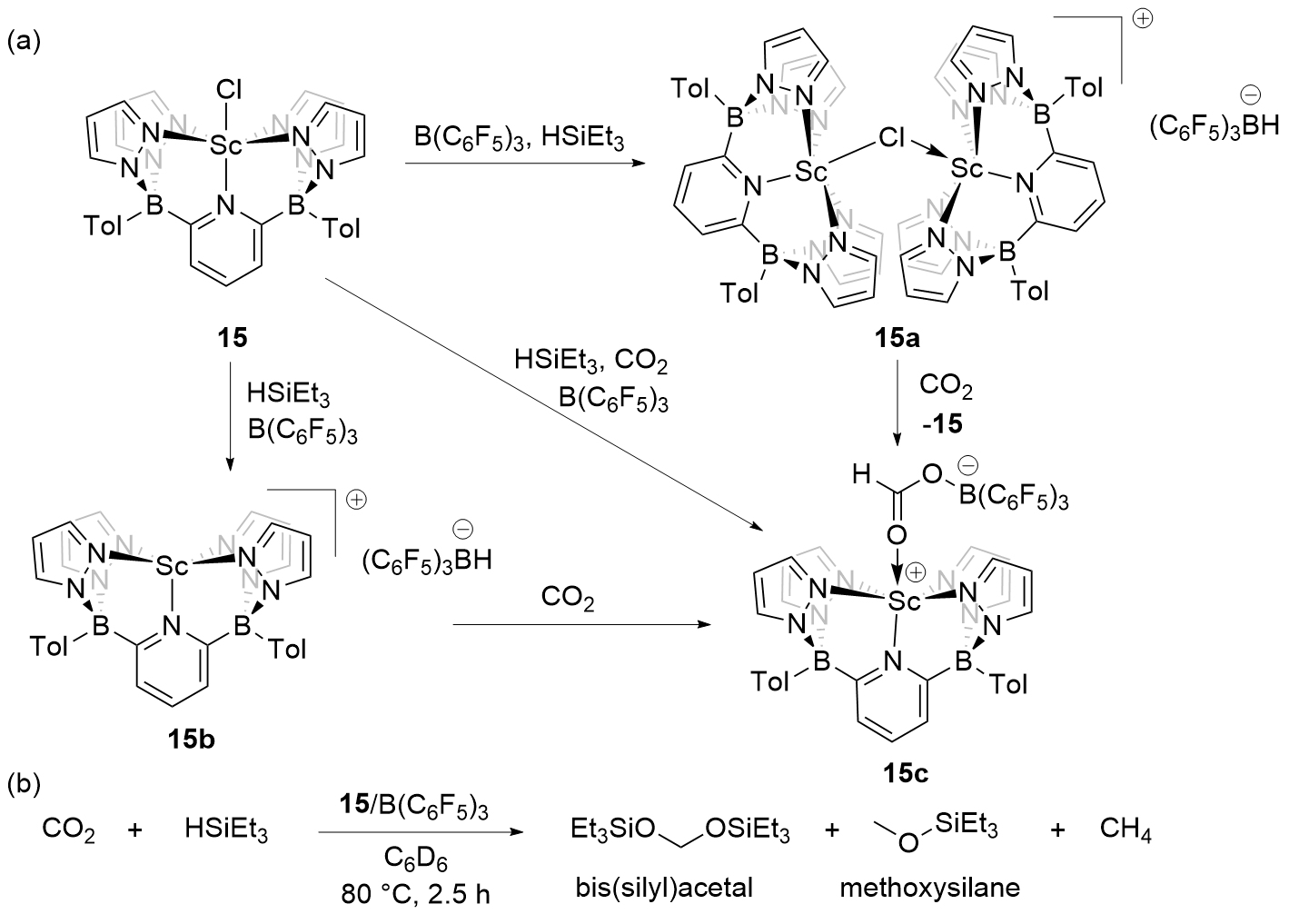
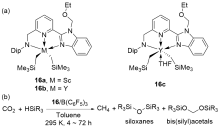
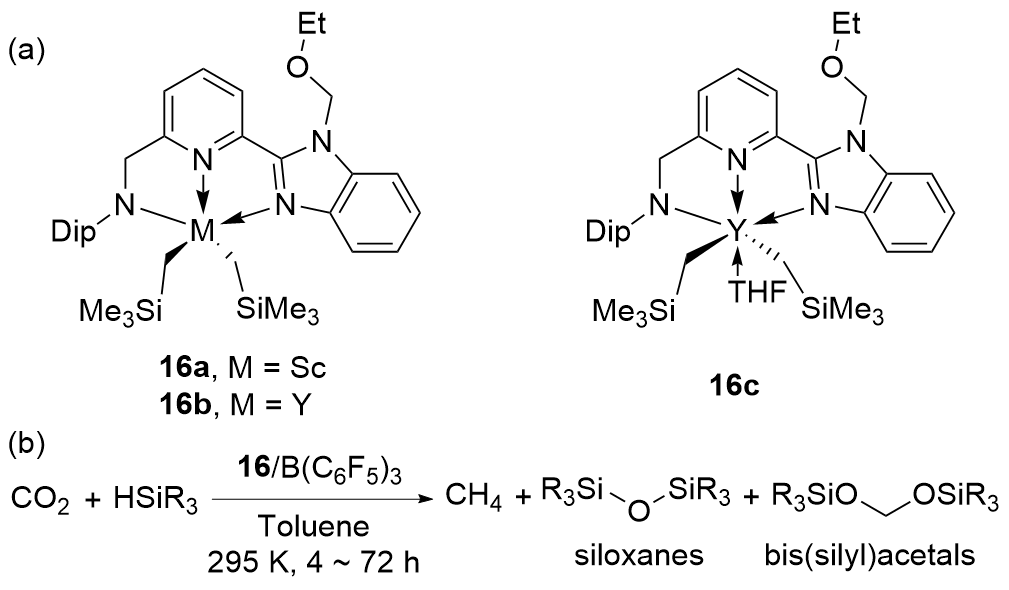
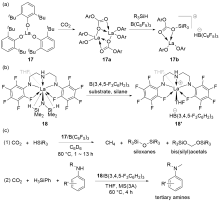

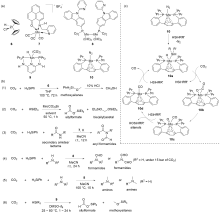
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 15/B(C6F5)3[ | HSiEt3 | 0.1 | — | C6D6 | 80 | 2.5 | Bis(silyl)acetals+ methoxysilanes+CH4 |
| 2 | 16/B(C6F5)3[ | HSiMe2Ph, H3SiPh, or HSiEt2Me | 0.1 | — | Toluene | 22 | 4~72 | Bis(silyl)acetals+silo- xanes+CH4 |
| 3 | 17/B(C6F5)3[ | HSiMePh2, H3SiPh, or HSiEt3 | 0.5 | — | C6D6 | 80 | 1~13 | Bis(silyl)acetals+silo- xanes+CH4 |
| 4a | 18/B(3,4,5-F3C6H2)3[ | H3SiPh | 0.1 | Anilines | THF | 60 | 4 | tertiary amines |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 15/B(C6F5)3[ | HSiEt3 | 0.1 | — | C6D6 | 80 | 2.5 | Bis(silyl)acetals+ methoxysilanes+CH4 |
| 2 | 16/B(C6F5)3[ | HSiMe2Ph, H3SiPh, or HSiEt2Me | 0.1 | — | Toluene | 22 | 4~72 | Bis(silyl)acetals+silo- xanes+CH4 |
| 3 | 17/B(C6F5)3[ | HSiMePh2, H3SiPh, or HSiEt3 | 0.5 | — | C6D6 | 80 | 1~13 | Bis(silyl)acetals+silo- xanes+CH4 |
| 4a | 18/B(3,4,5-F3C6H2)3[ | H3SiPh | 0.1 | Anilines | THF | 60 | 4 | tertiary amines |
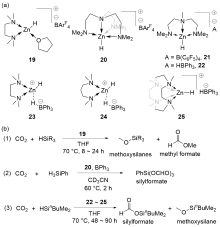



| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | Zn(OAc)2/phen[ | H2SiPh2 | 1 | — | CD3CN | r.t.~80 | 24 | Silylformates+bis(silyl)ace- tals+methoxysilanes+CH4 |
| 2 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Amines | MeCN | 25 | 4~24 | Formamidesa |
| 3 | Zn(OAc)2/phen[ | H2SiPh2 | 0.5 | Amines | MeCN | 120 | 24 | Tertiary amines |
| 4 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Amides | MeCN | 25~100 | 24 | Acyl formamides |
| 5 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Carbamates | MeCN | 25~100 | 24 | Formate formamides |
| 6 | ZnEt2[ | HSi(OEt)3 | 1 | — | Neat | 90 | 7 | Siloxanes+methoxysilanes |
| 7 | ZnEt2[ | HSi(OEt)3 | 1.5 | Secondary amines | Neat | 100 | 24 | Formamides |
| 8 | ZnEt2[ | HSi(OEt)3 | 1.5 | Primary amines | Neat | 100 | 24 | Urea derivatives |
| 9 | 19[ | HSiEtMe2 or HSinBuMe2 | 0.1 | — | THF | 70 | 8~24 | Methoxysilanes+methyl formate |
| 10b | 20[ | H3SiPh | 0.15 | — | CD3CN | 60 | 2 | silylformates |
| 11 | 22[ | HSinBuMe2 | 0.1 | — | THF | 70 | 90 | Methoxysilanes+silylformates |
| 12 | 23[ | HSinBuMe2 | 0.1 | — | THF | 70 | 90 | Methoxysilanes+silylformates |
| 13 | 24[ | HSinBuMe2 | 0.1 | — | THF | 70 | 48 | Methoxysilanes+silylformates |
| 14 | 24[ | HSinBuMe2 | 0.1 | — | THF | 70 | 72 | Methoxysilanes |
| 15 | 25[ | HSinBuMe2 | 0.1 | — | THF | 70 | 48 | Silylformates |
| 16 | 26[ | HSi[N(CH2CH2O)3] or HSi(OEt)3 | 0.1 | — | C6D6 | 100 | 24~264 | Methoxysilanes+silylformates |
| 17 | 27[ | HSi(OMe)3 or HSi(OEt)3 | 0.1 | — | C6D6 | r.t. | 72 | Silylformates |
| 18 | 28[ | HSi(OEt)3 | 0.1 | — | DMF | 60~80 | 24 | Silylformates |
| 19 | 29[ | H3SiPh | 0.1 | Anilines or indoles | Neat | 60~100 | 24~96 | Anilines or indoles derivatives |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | Zn(OAc)2/phen[ | H2SiPh2 | 1 | — | CD3CN | r.t.~80 | 24 | Silylformates+bis(silyl)ace- tals+methoxysilanes+CH4 |
| 2 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Amines | MeCN | 25 | 4~24 | Formamidesa |
| 3 | Zn(OAc)2/phen[ | H2SiPh2 | 0.5 | Amines | MeCN | 120 | 24 | Tertiary amines |
| 4 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Amides | MeCN | 25~100 | 24 | Acyl formamides |
| 5 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Carbamates | MeCN | 25~100 | 24 | Formate formamides |
| 6 | ZnEt2[ | HSi(OEt)3 | 1 | — | Neat | 90 | 7 | Siloxanes+methoxysilanes |
| 7 | ZnEt2[ | HSi(OEt)3 | 1.5 | Secondary amines | Neat | 100 | 24 | Formamides |
| 8 | ZnEt2[ | HSi(OEt)3 | 1.5 | Primary amines | Neat | 100 | 24 | Urea derivatives |
| 9 | 19[ | HSiEtMe2 or HSinBuMe2 | 0.1 | — | THF | 70 | 8~24 | Methoxysilanes+methyl formate |
| 10b | 20[ | H3SiPh | 0.15 | — | CD3CN | 60 | 2 | silylformates |
| 11 | 22[ | HSinBuMe2 | 0.1 | — | THF | 70 | 90 | Methoxysilanes+silylformates |
| 12 | 23[ | HSinBuMe2 | 0.1 | — | THF | 70 | 90 | Methoxysilanes+silylformates |
| 13 | 24[ | HSinBuMe2 | 0.1 | — | THF | 70 | 48 | Methoxysilanes+silylformates |
| 14 | 24[ | HSinBuMe2 | 0.1 | — | THF | 70 | 72 | Methoxysilanes |
| 15 | 25[ | HSinBuMe2 | 0.1 | — | THF | 70 | 48 | Silylformates |
| 16 | 26[ | HSi[N(CH2CH2O)3] or HSi(OEt)3 | 0.1 | — | C6D6 | 100 | 24~264 | Methoxysilanes+silylformates |
| 17 | 27[ | HSi(OMe)3 or HSi(OEt)3 | 0.1 | — | C6D6 | r.t. | 72 | Silylformates |
| 18 | 28[ | HSi(OEt)3 | 0.1 | — | DMF | 60~80 | 24 | Silylformates |
| 19 | 29[ | H3SiPh | 0.1 | Anilines or indoles | Neat | 60~100 | 24~96 | Anilines or indoles derivatives |

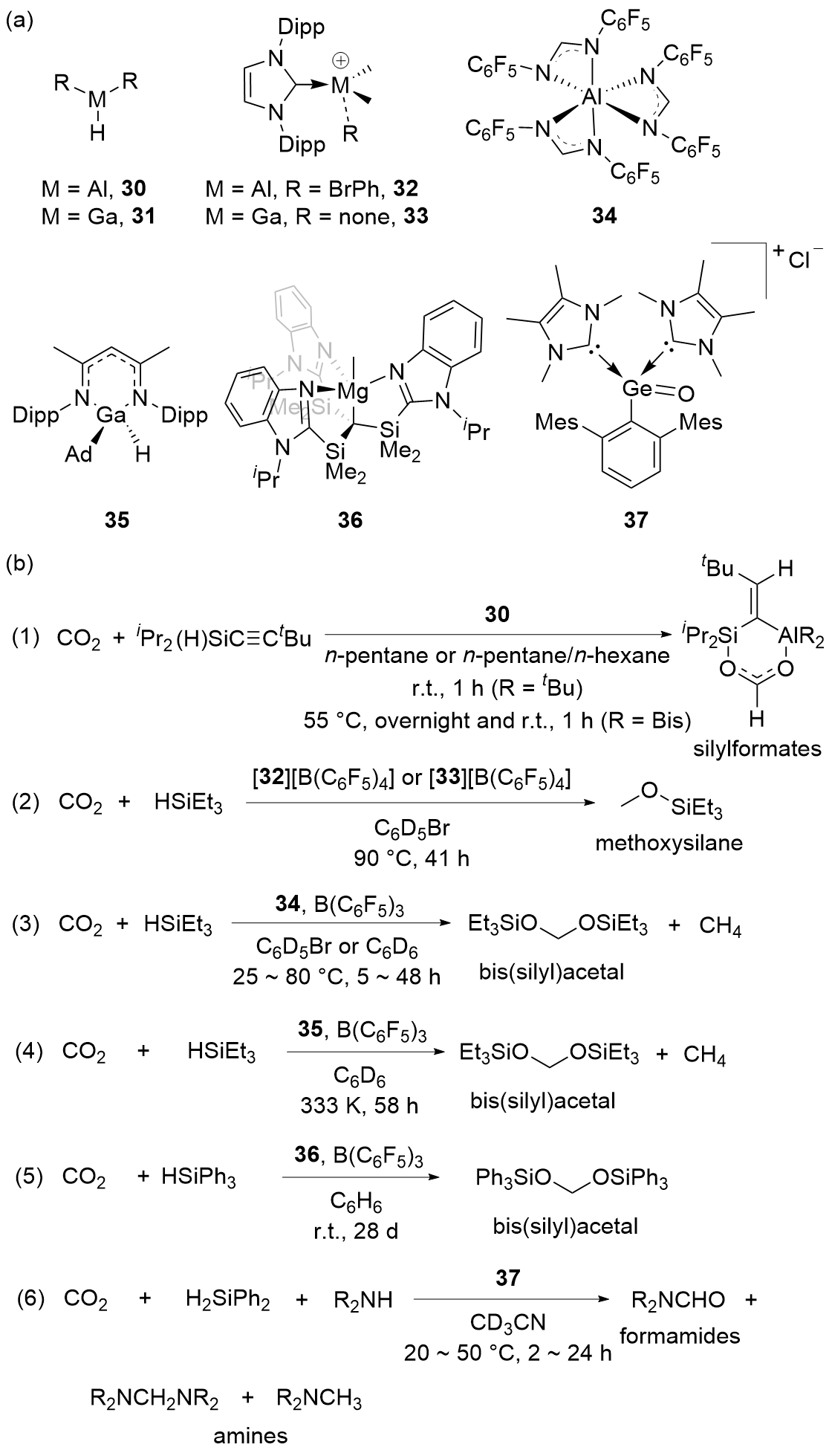
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 30[ | iPr2Si(H)C≡CtBu | 0.10 | — | n-Pentane or n- pentane/n-hexane | r.t.~55 | 1~12 | Silylformates |
| 2 | [32][B(C6F5)4][ | HSiEt3 | 0.15 | — | C6D5Br | 90 | 41 | Methoxysilanes |
| 3 | [33][B(C6F5)4][ | HSiEt3 | 0.15 | — | C6D5Br | 90 | 41 | Methoxysilanes |
| 4a | 34[ | HSiEt3 | 0.60 | — | C6D5Br or C6D6 | 25~80 | 5~48 | Bis(silyl)acetals+CH4 |
| 5a | 35[ | HSiEt3 | 0.20 | — | C6D6 | 60 | 58 | Bis(silyl)acetals+CH4 |
| 6a | 36[ | HSiPh3 | 0.10 | — | C6H6 | r.t. | 672 | Bis(silyl)acetals |
| 7 | 37[ | H2SiPh2 | 0.10 | Amines | CD3CN | 20~50 | 2~24 | Formamides+diami- nes+methyl amines |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 30[ | iPr2Si(H)C≡CtBu | 0.10 | — | n-Pentane or n- pentane/n-hexane | r.t.~55 | 1~12 | Silylformates |
| 2 | [32][B(C6F5)4][ | HSiEt3 | 0.15 | — | C6D5Br | 90 | 41 | Methoxysilanes |
| 3 | [33][B(C6F5)4][ | HSiEt3 | 0.15 | — | C6D5Br | 90 | 41 | Methoxysilanes |
| 4a | 34[ | HSiEt3 | 0.60 | — | C6D5Br or C6D6 | 25~80 | 5~48 | Bis(silyl)acetals+CH4 |
| 5a | 35[ | HSiEt3 | 0.20 | — | C6D6 | 60 | 58 | Bis(silyl)acetals+CH4 |
| 6a | 36[ | HSiPh3 | 0.10 | — | C6H6 | r.t. | 672 | Bis(silyl)acetals |
| 7 | 37[ | H2SiPh2 | 0.10 | Amines | CD3CN | 20~50 | 2~24 | Formamides+diami- nes+methyl amines |

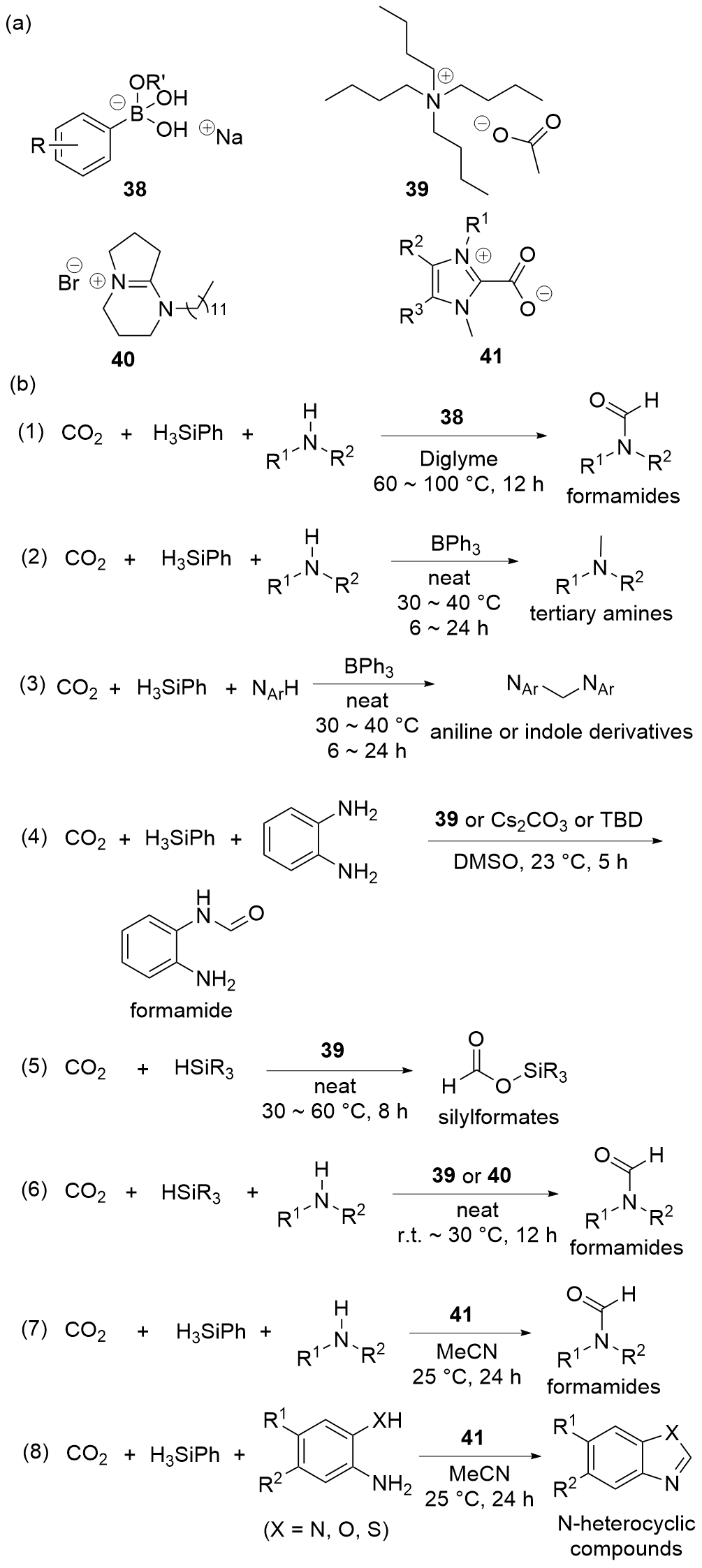
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 38[ | H3SiPh | 0.25 | Amines | Diglyme | 60~100 | 12 | Formamides |
| 2 | BPh3[ | H3SiPh | 0.1 | Amines | Neat | 30~40 | 6~24 | Tertiary amines |
| 3 | BPh3[ | H3SiPh | 0.1 | Anilines or indoles | Neat | 30~40 | 6~24 | Anilines or indoles derivatives |
| 4 | 39[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 5 | Cs2CO3[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 6 | TBD[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 7 | 39[ | HSiMe2Ph, HSiMePh2, HSiPh3, or H3SiPh | 0.1 | — | Neat | 30~60 | 8 | Silylformates |
| 8 | 39[ | H3SiPh or PMHS | 0.1 | Amines | Neat | 30 | 12 | Formamides |
| 9 | 40[ | H3SiPh | 0.5 | Amines | Neat | r.t. | 12 | Formamides |
| 10 | 41[ | H3SiPh | 0.1 | Amines | MeCN | 25 | 24 | Formamides |
| 11 | 41[ | H3SiPh | 0.1 | o-Phenylenediamines | MeCN | 25 | 24 | Benzimidazoles |
| 12 | 41[ | H3SiPh | 0.1 | o-Hydroxyaniline | MeCN | 25 | 24 | Benzoxazole |
| 13 | 41[ | H3SiPh | 0.1 | o-Mercaptonoaniline | MeCN | 25 | 24 | Benzothiazole |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 38[ | H3SiPh | 0.25 | Amines | Diglyme | 60~100 | 12 | Formamides |
| 2 | BPh3[ | H3SiPh | 0.1 | Amines | Neat | 30~40 | 6~24 | Tertiary amines |
| 3 | BPh3[ | H3SiPh | 0.1 | Anilines or indoles | Neat | 30~40 | 6~24 | Anilines or indoles derivatives |
| 4 | 39[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 5 | Cs2CO3[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 6 | TBD[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 7 | 39[ | HSiMe2Ph, HSiMePh2, HSiPh3, or H3SiPh | 0.1 | — | Neat | 30~60 | 8 | Silylformates |
| 8 | 39[ | H3SiPh or PMHS | 0.1 | Amines | Neat | 30 | 12 | Formamides |
| 9 | 40[ | H3SiPh | 0.5 | Amines | Neat | r.t. | 12 | Formamides |
| 10 | 41[ | H3SiPh | 0.1 | Amines | MeCN | 25 | 24 | Formamides |
| 11 | 41[ | H3SiPh | 0.1 | o-Phenylenediamines | MeCN | 25 | 24 | Benzimidazoles |
| 12 | 41[ | H3SiPh | 0.1 | o-Hydroxyaniline | MeCN | 25 | 24 | Benzoxazole |
| 13 | 41[ | H3SiPh | 0.1 | o-Mercaptonoaniline | MeCN | 25 | 24 | Benzothiazole |
| [1] |
Schuur E. A.; McGuire A. D.; Schadel C.; Grosse G.; Harden J. W.; Hayes D. J.; Hugelius G.; Koven C. D.; Kuhry P.; Lawrence D. M.; Natali S. M.; Olefeldt D.; Romanovsky V. E.; Schaefer K.; Turetsky M. R.; Treat C. C.; Vonk J. E. Nature 2015, 520, 171.
doi: 10.1038/nature14338 |
| [2] |
Canadell J. G.; Schulze E. D. Nat. Commun. 2014, 5, 5282.
doi: 10.1038/ncomms6282 pmid: 25407959 |
| [3] |
Hotchkiss J. H.; Werner B. G.; Lee E. Y. C. Compr. Rev. Food Sci. Food Saf. 2006, 5, 158.
doi: 10.1111/crfs.2006.5.issue-4 |
| [4] |
Cuomo R.; Sarnelli G.; Savarese M. F.; Buyckx M. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 683.
doi: 10.1016/j.numecd.2009.03.020 pmid: 19502016 |
| [5] |
Singh P.; Wani A. A.; Karim A. A.; Langowski H.-C. Int. J. Dairy Technol. 2012, 65, 161.
doi: 10.1111/idt.2012.65.issue-2 |
| [6] |
Pearson A. Int. J. Refrig. 2005, 28, 1140.
doi: 10.1016/j.ijrefrig.2005.09.005 |
| [7] |
Ramsey E.; Sun Q.; Zhang Z.; Zhang C.; Gou W. J. Environ. Sci. 2009, 21, 720.
doi: 10.1016/S1001-0742(08)62330-X |
| [8] |
Zhou Y.; Zhu R.; Wei G. Powder Technol. 2021, 389, 21.
doi: 10.1016/j.powtec.2021.05.003 |
| [9] |
You X.; He S.; Zhang M.; Zeng J.; Li L.; Wang Q.; Wang Q.; Li Y. Steel Res. Int. 2019, 91, 1900450.
doi: 10.1002/srin.v91.2 |
| [10] |
Maeda C.; Miyazaki Y.; Ema T. Catal. Sci. Technol. 2014, 4, 1482.
|
| [11] |
Ye R.-P.; Ding J.; Gong W.; Argyle M. D.; Zhong Q.; Wang Y.; Russell C. K.; Xu Z.; Russell A. G.; Li Q.; Fan M.; Yao Y.-G. Nat. Commun. 2019, 10, 5698.
doi: 10.1038/s41467-019-13638-9 |
| [12] |
Artz J.; Müller T. E.; Thenert K.; Kleinekorte J.; Meys R.; Sternberg A.; Bardow A.; Leitner W. Chem. Rev. 2017, 118, 434.
doi: 10.1021/acs.chemrev.7b00435 |
| [13] |
Chauvier C.; Cantat T. ACS Catal. 2017, 7, 2107.
doi: 10.1021/acscatal.6b03581 |
| [14] |
Kostera S.; Peruzzini M.; Gonsalvi L. Catalysts 2021, 11, 58.
doi: 10.3390/catal11010058 |
| [15] |
Fernández-Alvarez F. J.; Oro L. A. ChemCatChem 2018, 10, 4783.
|
| [16] |
Chen J.; McGraw M.; Chen E. Y. ChemSusChem 2019, 12, 4543.
doi: 10.1002/cssc.v12.20 |
| [17] |
Wang X.; Xia C.; Wu L. Green Chem. 2018, 20, 5415.
doi: 10.1039/C8GC03022G |
| [18] |
Zhang Y.; Zhang T.; Das S. Green Chem. 2020, 22, 1800.
doi: 10.1039/C9GC04342J |
| [19] |
Motokura K.; Pramudita R. A. Chem. Rec. 2019, 19, 1199.
doi: 10.1002/tcr.201800076 |
| [20] |
Fernández-Alvarez F. J.; Aitani A. M.; Oro L. A. Catal. Sci. Technol. 2014, 4, 611.
doi: 10.1039/C3CY00948C |
| [21] |
Iglesias M.; Fernández-Alvarez F. J.; Oro L. A. Coord. Chem. Rev. 2019, 386, 240.
doi: 10.1016/j.ccr.2019.02.003 |
| [22] |
Zhao S.; Liang H.-Q.; Hu X.-M.; Li S.; Daasbjerg K. Angew. Chem., Int. Ed. 2022, 61, e202204008.
|
| [23] |
Liu M.; Qin T.; Zhang Q.; Fang C.; Fu Y.; Lin B.-L. Sci. China: Chem. 2015, 58, 1524.
|
| [24] |
Li B.; Sortais J.-B.; Darcel C. RSC Adv. 2016, 6, 57603.
doi: 10.1039/C6RA10494K |
| [25] |
Liu X.; Li J.; Li N.; Li B.; Bu X.-H. Chin. J. Chem. 2021, 39, 440.
doi: 10.1002/cjoc.v39.2 |
| [26] |
Jiang Y.; Li G.; Chen Q.; Xu Z.; Lin S.; Guo G. Acta Chim. Sinica 2022, 80, 703 (in Chinese).
doi: 10.6023/A22010012 |
|
(蒋银龙, 李国超, 陈青松, 徐忠宁, 林姗姗, 郭国聪, 化学学报, 2022, 80, 703.)
doi: 10.6023/A22010012 |
|
| [27] |
Wang X.; Yang X.; Chen C.; Li H.; Huang Y.; Cao R. Acta Chim. Sinica 2022, 80, 22 (in Chinese).
doi: 10.6023/A21100455 |
|
(王旭生, 杨胥, 陈春辉, 李红芳, 黄远标, 曹荣, 化学学报, 2022, 80, 22.)
doi: 10.6023/A21100455 |
|
| [28] |
Addis D.; Das S.; Junge K.; Beller M. Angew. Chem., Int. Ed. 2011, 50, 6004.
doi: 10.1002/anie.v50.27 |
| [29] |
Wang H.; Dong Y.; Zheng C.; Sandoval C. A.; Wang X.; Makha M.; Li Y. Chem 2018, 4, 2883.
doi: 10.1016/j.chempr.2018.09.009 |
| [30] |
Yan F.; Bai J.-F.; Dong Y.; Liu S.; Li C.; Du C.-X.; Li Y. JACS Au 2022, 2, 2522.
doi: 10.1021/jacsau.2c00392 |
| [31] |
Dong Y.; Yang P.; Zhao S.; Li Y. Nat. Commun. 2020, 11, 4096.
doi: 10.1038/s41467-020-17939-2 |
| [32] |
Kunihiro K.; Heyte S.; Paul S.; Roisnel T.; Carpentier J. F.; Kirillov E. Chem. Eur. J. 2021, 27, 3997.
doi: 10.1002/chem.v27.12 |
| [33] |
Guzmán J.; Torguet A.; García-Orduña P.; Lahoz F. J.; Oro L. A.; Fernández-Alvarez F. J. J. Organomet. Chem. 2019, 897, 50.
doi: 10.1016/j.jorganchem.2019.06.010 |
| [34] |
Guzmán J.; Urriolabeitia A.; Padilla M.; García-Orduña P.; Polo V.; Fernández-Alvarez F. J. Inorg. Chem. 2022, 61, 20216.
doi: 10.1021/acs.inorgchem.2c03330 |
| [35] |
Guzmán J.; García-Orduña P.; Lahoz F. J.; Fernández-Alvarez F. J. RSC Adv. 2020, 10, 9582.
doi: 10.1039/D0RA00204F |
| [36] |
Ojeda-Amador A. I.; Munarriz J.; Alamán-Valtierra P.; Polo V.; Puerta-Oteo R.; Jiménez M. V.; Fernández-Alvarez F. J.; Pérez-Torrente J. J. ChemCatChem 2019, 11, 5524.
doi: 10.1002/cctc.201901687 |
| [37] |
Roa D. A.; Garcia J. J. New J. Chem. 2023, 47, 4504.
doi: 10.1039/D2NJ06204F |
| [38] |
González T.; García J. J. Polyhedron 2021, 203, 115242.
doi: 10.1016/j.poly.2021.115242 |
| [39] |
Chakraborty S.; Nath R.; Kumar Ray A.; Paul A.; Mandal S. K. Chem. Eur. J. 2022, 28, e202202710.
|
| [40] |
Huang Z.; Jiang X.; Zhou S.; Yang P.; Du C.-X.; Li Y. ChemSusChem 2019, 12, 3054.
doi: 10.1002/cssc.v12.13 |
| [41] |
Bertini F.; Glatz M.; Stöger B.; Peruzzini M.; Veiros L. F.; Kirchner K.; Gonsalvi L. ACS Catal. 2019, 9, 632.
doi: 10.1021/acscatal.8b04106 |
| [42] |
Buss J. A.; Shida N.; He T.; Agapie T. ACS Catal. 2021, 11, 13294.
doi: 10.1021/acscatal.1c02922 |
| [43] |
Song Z.; Liu J.; Xing S.; Shao X.; Li J.; Peng J.; Bai Y. Org. Biomol. Chem. 2023, 21, 832.
doi: 10.1039/D2OB01986H |
| [44] |
Yang F.; Saiki Y.; Nakaoka K.; Ema T. Adv. Synth. Catal. 2023, 365, 877.
doi: 10.1002/adsc.v365.6 |
| [45] |
Cramer H. H.; Chatterjee B.; Weyhermuller T.; Werlé C.; Leitner W. Angew. Chem., Int. Ed. 2020, 59, 15674.
doi: 10.1002/anie.v59.36 |
| [46] |
Cramer H. H.; Ye S.; Neese F.; Werlé C.; Leitner W. JACS Au 2021, 1, 2058.
doi: 10.1021/jacsau.1c00350 pmid: 34849511 |
| [47] |
Siddique M.; Boity B.; Rit A. Organometallics 2023, 42, 1395.
doi: 10.1021/acs.organomet.2c00670 |
| [48] |
Li W.-D.; Chen J.; Zhu D.-Y.; Xia J.-B. Chin. J. Chem. 2021, 39, 614.
doi: 10.1002/cjoc.v39.3 |
| [49] |
Beh D. W.; Piers W. E.; Gelfand B. S.; Lin J.-B. Dalton Trans. 2020, 49, 95.
doi: 10.1039/C9DT04323C |
| [50] |
Gurina G. A.; Kissel A. A.; Lyubov D. M.; Luconi L.; Rossin A.; Tuci G.; Cherkasov A. V.; Lyssenko K. A.; Shavyrin A. S.; Ob'edkov A. M.; Giambastiani G.; Trifonov A. A. Dalton Trans. 2020, 49, 638.
doi: 10.1039/c9dt04338a pmid: 31819930 |
| [51] |
Chang K.; del Rosal I.; Zheng X.; Maron L.; Xu X. Dalton Trans. 2021, 50, 7804.
doi: 10.1039/D1DT01074C |
| [52] |
Shinohara K.; Tsurugi H.; Mashima K. ACS Catal. 2022, 12, 8220.
doi: 10.1021/acscatal.2c01658 |
| [53] |
Zhang Q.; Fukaya N.; Fujitani T.; Choi J.-C. Bull. Chem. Soc. Jpn. 2019, 92, 1945.
doi: 10.1246/bcsj.20190203 |
| [54] |
Zhang Q.; Lin X.-T.; Fukaya N.; Fujitani T.; Sato K.; Choi J.-C. Green Chem. 2020, 22, 8414.
doi: 10.1039/D0GC02890H |
| [55] |
Du C.; Chen Y. Acta Chim. Sinica 2020, 78, 938 (in Chinese).
doi: 10.6023/A20060268 |
|
(杜重阳, 陈耀峰. 化学学报, 2020, 78, 938.)
doi: 10.6023/A20060268 |
|
| [56] |
Ritter F.; Spaniol T. P.; Douair I.; Maron L.; Okuda J. Angew. Chem., Int. Ed. 2020, 59, 23335.
doi: 10.1002/anie.v59.51 |
| [57] |
Huang X.; Zhang K.; Shao Y.; Li Y.; Gu F.-L.; Qu L.-B.; Zhao C.; Ke Z. ACS Catal. 2019, 9, 5279.
doi: 10.1021/acscatal.9b00879 |
| [58] |
Chambenahalli R.; Bhargav R. M.; McCabe K. N.; Andrews A. P.; Ritter F.; Okuda J.; Maron L.; Venugopal A. Chem. Eur. J. 2021, 27, 7391.
doi: 10.1002/chem.v27.26 |
| [59] |
Ritter F.; Morris L. J.; McCabe K. N.; Spaniol T. P.; Maron L.; Okuda J. Inorg. Chem. 2021, 60, 15583.
doi: 10.1021/acs.inorgchem.1c02207 |
| [60] |
Ruccolo S.; Amemiya E.; Shlian D. G.; Parkin G. Can. J. Chem. 2021, 99, 259.
doi: 10.1139/cjc-2020-0451 |
| [61] |
Ruccolo S.; Sambade D.; Shlian D. G.; Amemiya E.; Parkin G. Dalton Trans. 2022, 51, 5868.
doi: 10.1039/D1DT04156H |
| [62] |
Sattler W.; Shlian D. G.; Sambade D.; Parkin G. Polyhedron 2020, 187, 114542.
doi: 10.1016/j.poly.2020.114542 |
| [63] |
Sattler W.; Parkin G. Catal. Sci. Technol. 2014, 4, 1578.
doi: 10.1039/c3cy01065a |
| [64] |
Ruccolo S.; Sattler W.; Rong Y.; Parkin G. J. Am. Chem. Soc. 2016, 138, 14542.
pmid: 27779860 |
| [65] |
Ruccolo S.; Rauch M.; Parkin G. Organometallics 2018, 37, 1708.
doi: 10.1021/acs.organomet.8b00158 |
| [66] |
Shlian D. G.; Amemiya E.; Parkin G. Chem. Commun. 2022, 58, 4188.
doi: 10.1039/D1CC06963B |
| [67] |
Baalbaki H. A.; Shu J.; Nyamayaro K.; Jung H.-J.; Mehrkhodavandi P. Chem. Commun. 2022, 58, 6192.
doi: 10.1039/D2CC01498J |
| [68] |
Takaishi K.; Kosugi H.; Nishimura R.; Yamada Y.; Ema T. Chem. Commun. 2021, 57, 8083.
doi: 10.1039/D1CC03675K |
| [69] |
Tolzmann M.; Schürmann L.; Hepp A.; Uhl W.; Layh M. Eur. J. Inorg. Chem. 2020, 4024.
|
| [70] |
Bolley A.; Specklin D.; Dagorne S. Polyhedron 2021, 194, 114956.
doi: 10.1016/j.poly.2020.114956 |
| [71] |
Huang W.; Roisnel T.; Dorcet V.; Orione C.; Kirillov E. Organometallics 2020, 39, 698.
doi: 10.1021/acs.organomet.9b00853 |
| [72] |
Caise A.; Hicks J.; Ángeles Fuentes M.; Goicoechea J. M.; Aldridge S. Chem. Eur. J. 2021, 27, 2138.
doi: 10.1002/chem.v27.6 |
| [73] |
Rauch M.; Strater Z.; Parkin G. J. Am. Chem. Soc. 2019, 141, 17754.
doi: 10.1021/jacs.9b08342 |
| [74] |
Sarkar D.; Weetman C.; Dutta S.; Schubert E.; Jandl C.; Koley D.; Inoue S. J. Am. Chem. Soc. 2020, 142, 15403.
doi: 10.1021/jacs.0c06287 |
| [75] |
Jiang X.; Huang Z.; Makha M.; Du C.-X.; Zhao D.; Wang F.; Li Y. Green Chem. 2020, 22, 5317.
doi: 10.1039/D0GC01741H |
| [76] |
Murata T.; Hiyoshi M.; Maekawa S.; Saiki Y.; Ratanasak M.; Hasegawa J.; Ema T. Green Chem. 2022, 24, 2385.
doi: 10.1039/D1GC04599G |
| [77] |
Hulla M.; Nussbaum S.; Bonnin A. R.; Dyson P. J. Chem. Commun. 2019, 55, 13089.
doi: 10.1039/C9CC06156H |
| [78] |
Murata T.; Hiyoshi M.; Ratanasak M.; Hasegawa J.; Ema T. Chem. Commun. 2020, 56, 5783.
doi: 10.1039/D0CC01371D |
| [79] |
Li X.-Y.; Fu H.-C.; Liu X.-F.; Yang S.-H.; Chen K.-H.; He L.-N. Catal. Today 2020, 356, 563.
doi: 10.1016/j.cattod.2020.01.030 |
| [80] |
Yu Z.; Li Z.; Zhang L.; Zhu K.; Wu H.; Li H.; Yang S. Green Chem. 2021, 23, 5759.
doi: 10.1039/D1GC01897C |
| [1] | 廖旭, 王泽宇, 唐武飞, 林金清. 多孔有机聚合物用于化学固定二氧化碳的研究进展[J]. 有机化学, 2023, 43(8): 2699-2710. |
| [2] | 刘露, 张曙光, 胡仁威, 赵晓晓, 崔京南, 贡卫涛. 基于多羟基柱[5]芳烃的酚醛多孔聚合物合成及CO2催化转化[J]. 有机化学, 2023, 43(8): 2808-2814. |
| [3] | 宋姿洁, 刘俊, 白赢, 厉嘉云, 彭家建. 利用硅氢加成反应催化转化二氧化碳研究进展[J]. 有机化学, 2023, 43(6): 2068-2080. |
| [4] | 刘双, 邹亮华, 王晓明. 均相钴催化氨硼烷的脱氢及转移氢化反应的研究进展[J]. 有机化学, 2023, 43(5): 1713-1725. |
| [5] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [6] | 潘永周, 蒙秀金, 王迎春, 何慕雪. 电化学固定CO2构建羧酸衍生物的研究进展[J]. 有机化学, 2023, 43(4): 1416-1434. |
| [7] | 刘桂杰, 付正强, 陈飞, 徐彩霞, 李敏, 刘宁. N-杂环卡宾-吡啶锰配合物/四丁基碘化铵催化CO2和环氧化物合成环状碳酸酯[J]. 有机化学, 2023, 43(2): 629-635. |
| [8] | 孙伟, 朱守非. 铁系金属催化烯烃与三级硅烷的硅氢化反应研究进展[J]. 有机化学, 2023, 43(10): 3339-3351. |
| [9] | 冯向青, 杜海峰. B(C6F5)3催化不饱和烃的硅化反应[J]. 有机化学, 2023, 43(10): 3544-3557. |
| [10] | 陈飞, 陶晟, 刘宁, 代斌. CNN型双核Cu(I)配合物室温催化固定CO2的直接羧基化反应[J]. 有机化学, 2022, 42(8): 2471-2480. |
| [11] | 黄燕, 张谦, 詹乐武, 侯静, 李斌栋. 可见光诱导甲酸盐参与的烯烃氢羧化反应[J]. 有机化学, 2022, 42(8): 2568-2573. |
| [12] | 徐勇, 张永兴, 胡佳, 陈宬, 原晔, Francis Verpoort. ZnO/离子液体体系催化常压二氧化碳合成β-羰基氨基甲酸酯[J]. 有机化学, 2022, 42(8): 2542-2550. |
| [13] | 管怡雯, 常克俭, 孙千林, 徐信. 基于稀土金属路易斯酸碱对化学的研究进展[J]. 有机化学, 2022, 42(5): 1326-1335. |
| [14] | 朱有财, 丁欣欣, 孙莉, 刘振. CO2/C2H4耦合制备丙烯酸及其衍生物的研究进展[J]. 有机化学, 2022, 42(4): 965-977. |
| [15] | 王鹏, 杨妲, 刘欢. 1,3-二烯烃的羰基化反应研究进展[J]. 有机化学, 2021, 41(9): 3379-3389. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||