Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (3): 1153-1160.DOI: 10.6023/cjoc202009056 Previous Articles Next Articles
ARTICLES
兰新婵1, 王丽丽1,*( ), 段征1,*(
), 段征1,*( ), François Mathey1
), François Mathey1
收稿日期:2020-09-28
修回日期:2020-11-03
发布日期:2020-11-12
通讯作者:
王丽丽, 段征
基金资助:
Xinchan Lan1, Lili Wang1,*( ), Zheng Duan1,*(
), Zheng Duan1,*( ), François Mathey1
), François Mathey1
Received:2020-09-28
Revised:2020-11-03
Published:2020-11-12
Contact:
Lili Wang, Zheng Duan
About author:Supported by:Share
Xinchan Lan, Lili Wang, Zheng Duan, François Mathey. Synthesis of 2-Pyrenylphosphines via Phospho-Fries Rearrangement[J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1153-1160.
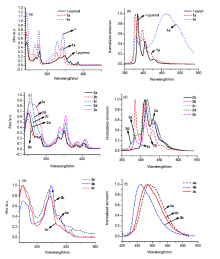

| Compound | λabsa/nm | λex/nm | λema/nm | τb/ns | Φfc | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Pyrenol | 335, 347, 364, 384 | 280 | 385, 405, 428 | 14.0 | 0.38 | ||||
| 1a | 326, 342 | 280 | 378, 398, 418 | 25.1 | 0.23 | ||||
| 1b | 326, 342 | 280 | 379, 398, 418 | 23.8 | 0.19 | ||||
| 1c | 326, 342 | 280 | 378, 397, 417 | 27.0 | 0.23 | ||||
| 1d | 329, 345 | 280 | 380, 399, 419 | 16.9 | 0.23 | ||||
| Compound | λabsa/nm | λex/nm | λema/nm | τb/ns | Φfc | ||||
| 1e | 328, 344 | 280 | 379, 398, 462 | 24.8 | 0.16 | ||||
| 2a | 335, 351, 383, 405 | 280 | 411, 433, 456 | 9.1 | 0.39 | ||||
| 2b | 335, 350, 384, 405 | 280 | 385, 408, 431, 457 | 10.6 | 0.39 | ||||
| 2c | 335, 351, 387, 408 | 280 | 415,438, 463 | 9.1 | 0.40 | ||||
| 2d | 338, 354, 385, 407 | 280 | 397, 418, 439 | 7.8 | 0.004 | ||||
| 2e | 338, 355, 386, 409 | 280 | 386, 418, 439 | 2.0 | 0.10 | ||||
| 4a | 254, 342 | 310 | 471 | 7.7 | 0.04 | ||||
| 4b | 252, 348 | 330 | 461 | 2.7 | 0.11 | ||||
| 4c | 249, 345 | 310 | 463 | 3.4 | 0.13 | ||||
| Compound | λabsa/nm | λex/nm | λema/nm | τb/ns | Φfc | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Pyrenol | 335, 347, 364, 384 | 280 | 385, 405, 428 | 14.0 | 0.38 | ||||
| 1a | 326, 342 | 280 | 378, 398, 418 | 25.1 | 0.23 | ||||
| 1b | 326, 342 | 280 | 379, 398, 418 | 23.8 | 0.19 | ||||
| 1c | 326, 342 | 280 | 378, 397, 417 | 27.0 | 0.23 | ||||
| 1d | 329, 345 | 280 | 380, 399, 419 | 16.9 | 0.23 | ||||
| Compound | λabsa/nm | λex/nm | λema/nm | τb/ns | Φfc | ||||
| 1e | 328, 344 | 280 | 379, 398, 462 | 24.8 | 0.16 | ||||
| 2a | 335, 351, 383, 405 | 280 | 411, 433, 456 | 9.1 | 0.39 | ||||
| 2b | 335, 350, 384, 405 | 280 | 385, 408, 431, 457 | 10.6 | 0.39 | ||||
| 2c | 335, 351, 387, 408 | 280 | 415,438, 463 | 9.1 | 0.40 | ||||
| 2d | 338, 354, 385, 407 | 280 | 397, 418, 439 | 7.8 | 0.004 | ||||
| 2e | 338, 355, 386, 409 | 280 | 386, 418, 439 | 2.0 | 0.10 | ||||
| 4a | 254, 342 | 310 | 471 | 7.7 | 0.04 | ||||
| 4b | 252, 348 | 330 | 461 | 2.7 | 0.11 | ||||
| 4c | 249, 345 | 310 | 463 | 3.4 | 0.13 | ||||
| [1] |
(a) Figueira-Duarte, T. M.; Müllen, K. Chem. Rev. 2011, 111, 7260.
pmid: 31909994 |
|
(b) Lorbach, D.; Wagner, M.; Baumgarten, M.; Müllen, K. Chem. Commun. 2013, 49, 10578.
pmid: 31909994 |
|
|
(c) Zhang, S. Q.; Qiao, X. L.; Chen, Y.; Wang, Y. Y.; Edkins, R. M.; Liu, Z. Q.; Li, H. X.; Fang, Q. Org. Lett. 2014, 16, 342.
pmid: 31909994 |
|
|
(d) Mateo-Alonso, A. Chem. Soc. Rev. 2014, 43, 6311.
pmid: 31909994 |
|
|
(e) Cariati, E.; Botta, C.; Danelli, S. G.; Forni, A.; Giaretta, A.; Giovanella, U.; Lucenti, E.; Marinotto, D.; Righetto, S. Ugo, R. Chem. Commun. 2014, 50, 14225.
pmid: 31909994 |
|
|
(f) Liu, J. Z.; Narita, A.; Osella, S.; Zhang, W.; Schollmeyer, D.; Beljonne, D.; Feng, X. L.; Müllen, K. J. Am. Chem. Soc. 2016, 138, 2602.
doi: 10.1021/jacs.5b10399 pmid: 31909994 |
|
|
(g) Krzeszewski, M.; Sahara, K.; Poronik, Y. M.; Kubo, T.; Gryko, D. T. Org. Lett. 2018, 20, 1517.
pmid: 31909994 |
|
|
(h) Fu, C. X.; Luo, S.; Li, Z. Z..; Ai, X.; Pang, Z. G.; Li, C.; Chen, K.; Zhou, L.; Li, F.; Huang, Y.; Lu, Z. Y. Chem. Commun. 2019, 55, 6317.
pmid: 31909994 |
|
|
(i) Ascherl, L.; Evans, E. W.; Gorman, J.; Orsborne, S.; Bessinger, D.; Bein, T.; Friend, R. H.; Auras, F. J. Am. Chem. Soc. 2019, 141, 15693.
pmid: 31909994 |
|
|
(j) Shao, J.-Y.; Yang, N.; Guo, W.; Cui, B.-B.; Chen, Q.; Zhong, Y.-W. Chem. Commun. 2019, 55, 13406.
pmid: 31909994 |
|
|
(k) Takaishi, K.; Iwachidoo, K.; Ema, T. J. Am. Chem. Soc. 2020, 142, 1774.
pmid: 31909994 |
|
|
(l) Wang, B. B.; Zhang, F.; Wang, S. K.; Yang, R. J.; Chen, C. C.; Zhao, W. Chem. Commun. 2020, 56, 2598.
pmid: 31909994 |
|
|
(m) Chen, Y.; Wang, Y. Y.; Yu, P.; Liu, Z. Q.; Fang, Q. Chin. J. Org. Chem. 2012, 32, 589. (in Chinese).
pmid: 31909994 |
|
|
(陈莹, 王园园, 于萍, 刘志强, 方奇, 有机化学, 2012, 32, 589.)
pmid: 31909994 |
|
|
(n) Zhong, K. L.; Guo, B. F.; Zhou, X.; Cai, K. D.; Tang, L. J.; Jin, L. Y. Prog. Chem. 2015, 27, 1230. (in Chinese).
pmid: 31909994 |
|
|
(钟克利, 郭宝峰, 周雪, 蔡克迪, 汤立军, 金龙一, 化学进展, 2015, 27, 1230.)
pmid: 31909994 |
|
|
(o) Gong, Y. B.; Zhan, X. J.; Li, Q. Q.; Li, Z. Sci. China: Chem. 2016, 59, 1623.
pmid: 31909994 |
|
| [2] |
(a) Hu, J.; Yip, J. H. K.; Ma, D.-L.; Wong, K.-Y.; Chung, W.-H. Organometallics 2009, 28, 51.
pmid: 30475600 |
|
(b) Hu, J. A.; Nguyen, M.-H.; Yip, J. H. K. Inorg. Chem. 2011, 50, 7429.
pmid: 30475600 |
|
|
(c) Deng, M.; Schley, N. D.; Ung, G. Inorg. Chem. 2018, 57, 15399.
pmid: 30475600 |
|
| [3] |
(a) Chua, C. J.; Ren, Y.; Baumgartner, T. Organometallics 2012, 31, 2425.
pmid: 28762408 |
|
(b) Behm, K.; Essner, J. B.; Barnes, C. L.; Baker, G. A.; Walensky, J. R. Dalton Trans. 2017, 46, 10867.
pmid: 28762408 |
|
| [4] |
(a) Akasaka, K.; Suzuki, T.; Ohrui, H.; Meguro, H. Anal. Lett. 1987, 20, 731.
pmid: 8898309 |
|
(b) Akasaka, K.; Ohata, A.; Ohrui, H.; Meguro, H. J. Chromatogr. B 1995, 665, 37.
pmid: 8898309 |
|
|
(c) Ohshima, T.; Hopia, A.; German, J. B.; Frankel, E. N. Lipids 1996, 31, 1091.
pmid: 8898309 |
|
|
(d) Shioji, K.; Oyama, Y.; Okuma, K.; Nakagawa, H. Bioorg. Med. Chem. Lett. 2010, 20, 3911.
pmid: 8898309 |
|
|
(e) Fang, Q. Y.; Li, J. W.; Li, S. Y.; Duan, R. H.; Wang, S. Q.; Yi, Y. P.; Guo, X. D.; Qian, Y.; Huang, W.; Yang, G. Q. Chem. Commun. 2017, 53, 5702.
pmid: 8898309 |
|
|
(f) Xing, C.; Liu, J. X.; Chen, F.; Li, Y. Y.; Lv, C. J.; Peng, Q. C.; Hou, H. W.; Li, K. Chem. Commun. 2020, 56, 4220.
doi: 10.1039/D0CC01031F pmid: 8898309 |
|
| [5] |
(a) Oyamada, T.; Sasabe, H.; Adachi, C.; Murase, S.; Tominaga, T.; Maeda, C. Appl. Phys. Lett. 2005, 86, 033503.
pmid: 29170548 |
|
(b) Matsushima, T.; Adachi, C. Thin Solid Films 2008, 516, 4288.
pmid: 29170548 |
|
|
(c) Matsushima, T.; Adachi, C. J. Appl. Phys. 2008, 103, 034501.
doi: 10.1063/1.2836972 pmid: 29170548 |
|
|
(d) Mallesham, G.; Swetha, C.; Niveditha, S.; Mohanty, M. E.; Babu, N. J.; Kumar, A.; Bhanuprakash, K.; Rao, V. J. J. Mater. Chem. C 2015, 3, 1208.
pmid: 29170548 |
|
|
(e) Lin, X.; Wegner, B.; Lee, K. M.; Fusella, M. A.; Zhang, F. Y.; Moudgil, K.; Rand, B. P.; Barlow, S.; Marder, S. R.; Koch, N.; Kahn, A. Nat. Mater. 2017, 16, 1209.
pmid: 29170548 |
|
| [6] |
Qiu, L. Q.; Hu, W.; Wu, D.; Duan, Z.; Mathey, F. Org. Lett. 2018, 20, 7821.
pmid: 30520641 |
| [7] |
(a) Melvin, L. S. Tetrahedron Lett. 1981, 22, 3375.
pmid: 32319779 |
|
(b) Heinicke, J.; Böhle, I.; Tzschach, A. J. Organomet. Chem. 1986, 317, 11.
pmid: 32319779 |
|
|
(c) Dhawan, B.; Redmore, D. J. Org. Chem. 1991, 56, 833.
pmid: 32319779 |
|
|
(d) Li, S. S.; Wang, G. Q. Phosphorus Sulfur Relat. Elem. 1991, 61, 119.
pmid: 32319779 |
|
|
(e) Paladino, J.; Guyard, C.; Thurieau, C.; Fauchère, J.-L. Helv. Chim. Acta 1993, 76, 2465.
pmid: 32319779 |
|
|
(f) Whisler, M. C.; MacNeil, S.; Snieckus, V.; Beak, P. Angew. Chem.. Int. Ed. 2004, 43, 2206.
pmid: 32319779 |
|
|
(g) Jayasundera, K. P.; Watson, A. J.; Taylor, C. M. Tetrahedron Lett. 2005, 46, 4311.
pmid: 32319779 |
|
|
(h) Yilmaz, E.; Bier, D.; Guillory, X.; Briels, J.; Ruiz-Blanco, Y. B.; Sanchez-Garcia, E.; Ottmann, C.; Kaiser, M. Chem.-Eur. J. 2018, 24, 13807.
pmid: 32319779 |
|
|
(i) Korb, M.; Lang, H. Chem. Soc. Rev. 2019, 48, 2829.
pmid: 32319779 |
|
|
(j) Patel, J. J.; Blackburn, T.; Alessi, M.; Sawinski, H.; Snieckus, V. Org. Lett. 2020, 22, 3860.
pmid: 32319779 |
|
|
(k) Taylor, C. M.; Watson, A. J. Curr. Org. Chem. 2004, 8, 623.
doi: 10.2174/1385272043370717 pmid: 32319779 |
|
| [8] |
Kenner, G. W.; Williams, N. R. J. Chem. Soc. 1955,522.
pmid: 18127479 |
| [9] |
(a) Heinicke, J.; Tzschach, A. Z. Chem. 1980, 20, 342.
|
|
(b) Heinicke, J.; Tzschach, A. Phosphorus Sulfur Relat. Elem. 1985, 25, 345.
|
|
| [10] |
(a) Washington, M. P.; Gudimetla, V. B.; Laughlin, F. L.; Deligonul, N.; He, S.; Payton, J. L.; Simpson, M. C.; Protasiewicz, J. D. J. Am. Chem. Soc. 2010, 132, 4566.
doi: 10.1021/ja1009426 pmid: 23989307 |
|
(b) Washington, M. P.; Payton, J. L.; Simpson, M. C.; Protasiewicz, J. D. Organometallics 2011, 30, 1975.
pmid: 23989307 |
|
|
(c) Laughlin, F.Li; Deligonul, N.; Rheingold, A. L.; Golen, J. A.; Laughlin, B. J.; Smith, R. C.; Protasiewicz, J. D. Organometallics 2013, 32, 7116. 888b2ca0-aaca-41d3-9521-80be05c0be74
doi: 10.1021/om400838g pmid: 23989307 |
|
|
(d) Wu, S. S.; Rheingold, A. L.; Protasiewicz, J. D. Chem. Commun. 2014, 50, 11036.
pmid: 23989307 |
|
|
(e) Laughlin, F. L.; Rheingold, A. L.; Deligonul, N.; Laughlin, B. J.; Smith, R. C.; Higham, L. J.; Protasiewicz, J. D. Dalton Trans. 2012, 41, 12016.
pmid: 23989307 |
|
|
(f) Wu, S. S.; Deligonal, N.; Protasiewicz, J. D. Dalton Trans. 2013, 42, 14866.
pmid: 23989307 |
|
| [11] |
Yesilot, S.; Cosut, B.; Alidagi, H. A.; Hacivelioglu, F.; Oezpinar, G. A.; Kilic, A. Dalton Trans. 2014, 43, 3428.
pmid: 24452362 |
| [1] | Yatong Fu, Chaofan Sun, Dan Zhang, Chengguo Jin, Juyou Lu. Recent Progress in B—H Bond Functionalization of nido-Carboranes [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 438-447. |
| [2] | Jian Zhang, Wanjie Liang, Yi Yang, Fachao Yan, Hui Liu. Regiocontrollable Difunctionalization of N-Allenamines [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 335-348. |
| [3] | Yanshuo Zhu, Hongyan Wang, Penghua Shu, Ke'na Zhang, Qilin Wang. Recent Advances on Alkoxy Radicals-Mediated C(sp3)—H Bond Functionalization via 1,5-Hydrogen Atom Transfer [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 1-17. |
| [4] | Jianghu Dong, Liangming Xuan, Chi Wang, Chenxi Zhao, Haifeng Wang, Qiongjiao Yan, Wei Wang, Fen'er Chen. Recent Advances in Visible-Light-Induced C(3)—H Functionalization of Quinoxalinones under Transition-Metal-Free or Photocatalyst-Free [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 111-136. |
| [5] | Yingjie Liu, Gangqing Shi, Ge Chou, Xin Zhang, Dongxue Song, Ning Chen, Miao Yu, Ying Xu. Progress of α-Position Functionalization of Ethers under Photo/Electrocatalysis [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2664-2681. |
| [6] | Yuchao Wang, Jinbiao Liu, Zhitao He. Palladium-Catalyzed Asymmetric Hydrofunctionalizations of Conjugated Dienes [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2614-2627. |
| [7] | Xiaoping Xu, Yifei Zhang, Xiaoyu Mo, Jun Jiang. Rh-Catalyzed C—H Functionalization Reaction between 3-Diazoindolin-2-imines and Pyrazolones for the Construction of 3-Pyrazolyl Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2519-2527. |
| [8] | Jiamin Ma, Jiaoxiong Li, Qiansen Meng, Xianghua Zeng. Advances on the Radical Sulfonation of Alkynes [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2040-2052. |
| [9] | Rongbin Cai, Bing Li, Qi Zhou, Longyi Zhu, Jun Luo. Synthesis of 4,8,9,10-Tetrafunctionalized 2-Azaadamantanes and Their 2-Azaprotoadamantane Skeleton Isomers [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2217-2225. |
| [10] | Linsheng Bai, Peng Hong, Anguo Ying. Research Progress of Functional Polyacrylonitrile Fiber in Promoting Organic Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1241-1270. |
| [11] | Yannian Pan, Xiao Qin, Chengkai Yuan, Yi Lu. Application of Ligands in Cp*Rh(III)-Catalyzed C—H Bond Functionalization Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 924-948. |
| [12] | Hongyu Hou, Yuanyuan Cheng, Bin Chen, Chenho Tung, Lizhu Wu. α-Acylation of Olefins via Photocatalysis [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1012-1022. |
| [13] | Luomo Li, Xiaohui Yang. Recent Advances in Ionic Transfer Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1036-1044. |
| [14] | Ning Liu, Xiaodan Cuan, Hui Li, Xiyan Duan. Progress in the Study of α-Functionalization of Enaminone [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 602-621. |
| [15] | Fang Wang, Lei Wang. Recent Advances in Functionalization of Aromatic C(sp2)—H Bonds Based on N-Nitroso Direction [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4157-4167. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||