Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (3): 959-968.DOI: 10.6023/cjoc202009007 Previous Articles Next Articles
REVIEWS
曾志刚a,b, 桑贤轲a, 袁波c, 吴鸣虎a,b,*( ), 张武元c,*(
), 张武元c,*( )
)
收稿日期:2020-09-02
修回日期:2020-09-26
发布日期:2020-10-15
通讯作者:
吴鸣虎, 张武元
基金资助:
Zhigang Zenga,b, Xianke Sanga, Bo Yuanc, Minghu Wua,b,*( ), Wuyuan Zhangc,*(
), Wuyuan Zhangc,*( )
)
Received:2020-09-02
Revised:2020-09-26
Published:2020-10-15
Contact:
Minghu Wu, Wuyuan Zhang
About author:Supported by:Share
Zhigang Zeng, Xianke Sang, Bo Yuan, Minghu Wu, Wuyuan Zhang. Advances of Haloperoxidases-Catalyzed Green Halogenation Reactions[J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 959-968.
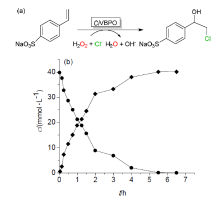
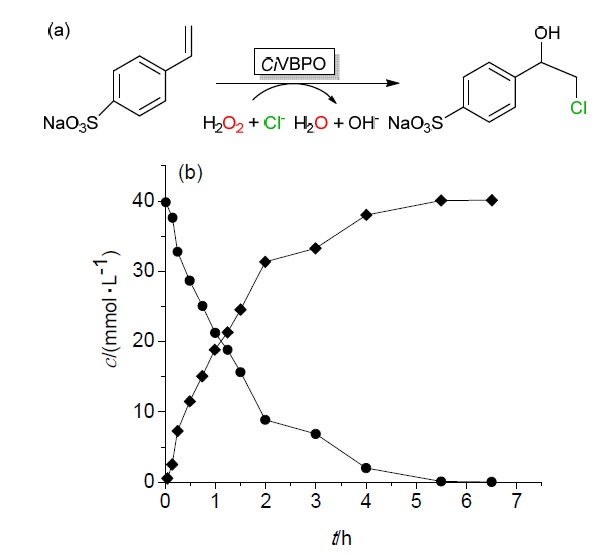
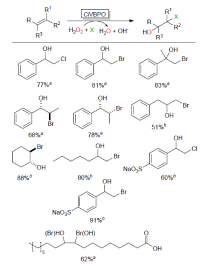
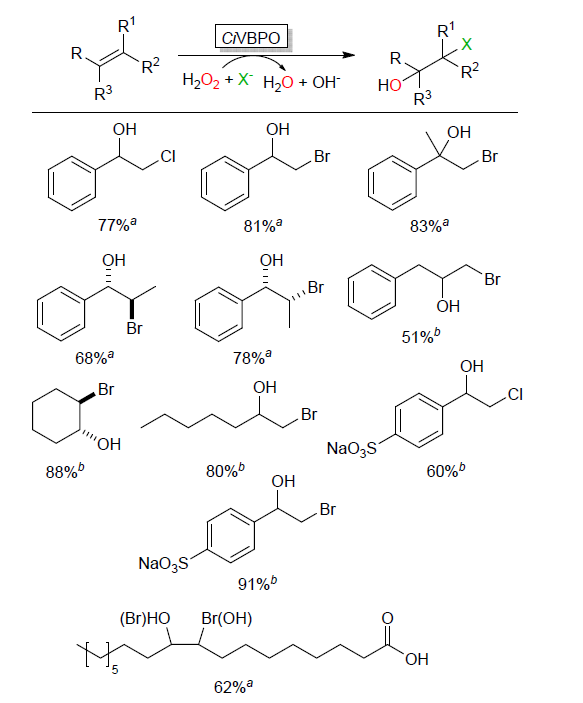
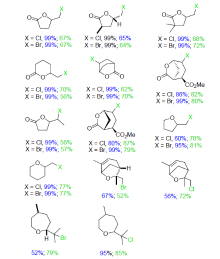
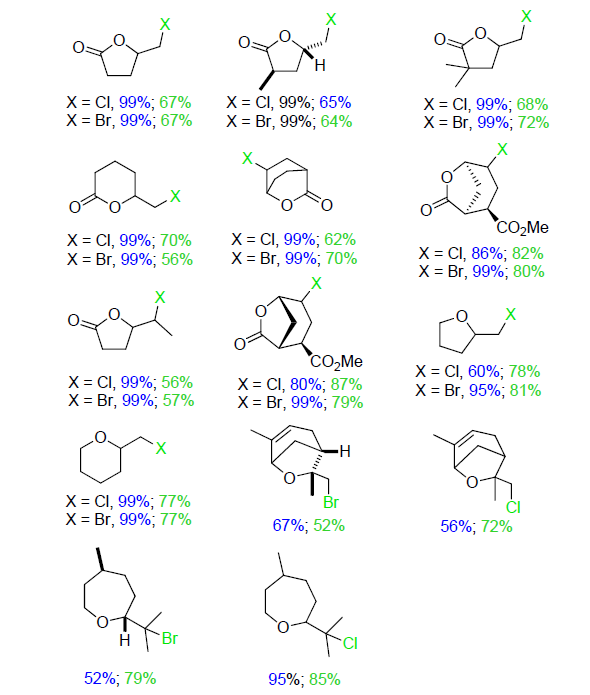
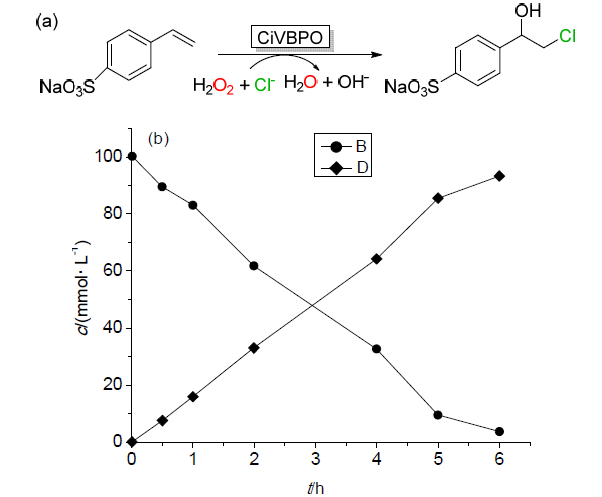
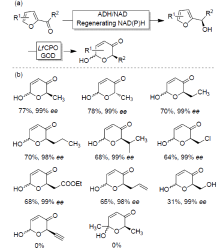
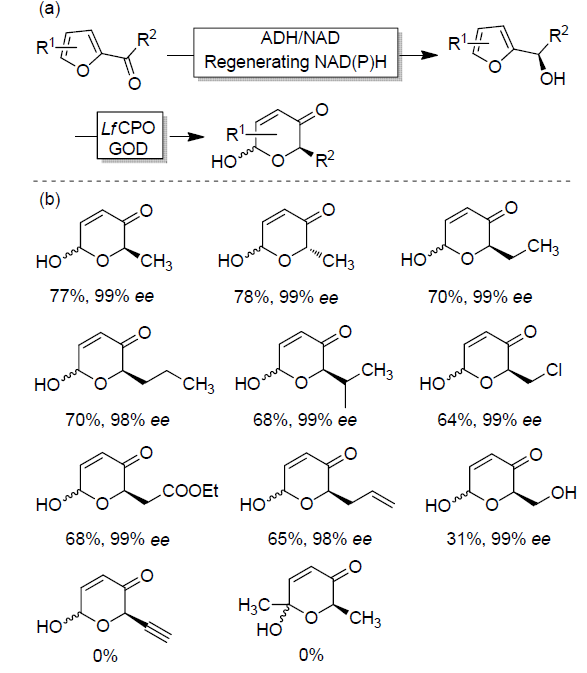
| Catalytic reaction | Catalyst | c/(mmol?L–1) | TONa/(mol?mol–1) | TOFa/s |
|---|---|---|---|---|
| | CiVCPO[ [VVO(OMe)(MeOH)][ | 20 396 | 198000 7117 | 55 1 |
| | CiVCPO[ NH4VO3[ NH4VO3[ None (substrates and NBS only)[ | 36 50 9 61 | 360000 0.5 0.9 n.a. | 15 n.a. 0.00006 n.a. |
| | CiVCPO[ $\text{WO}_{\text{4}}^{2}$ loaded on [Ni,Al]-LDH[ | 5 125 | 277778 28 | 15 0.0005 |
| Catalytic reaction | Catalyst | c/(mmol?L–1) | TONa/(mol?mol–1) | TOFa/s |
|---|---|---|---|---|
| | CiVCPO[ [VVO(OMe)(MeOH)][ | 20 396 | 198000 7117 | 55 1 |
| | CiVCPO[ NH4VO3[ NH4VO3[ None (substrates and NBS only)[ | 36 50 9 61 | 360000 0.5 0.9 n.a. | 15 n.a. 0.00006 n.a. |
| | CiVCPO[ $\text{WO}_{\text{4}}^{2}$ loaded on [Ni,Al]-LDH[ | 5 125 | 277778 28 | 15 0.0005 |
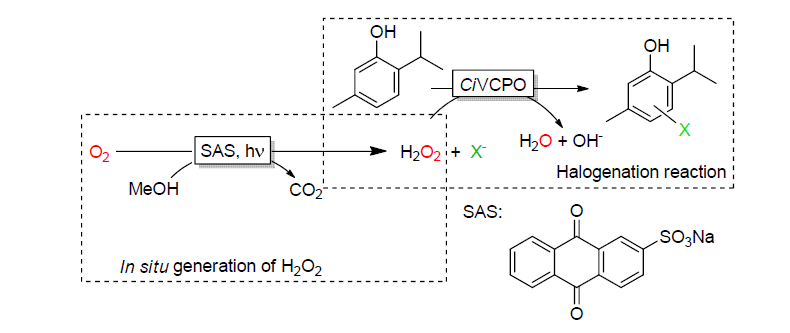
| [1] |
https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/Top%20200%20Drugs%20By%20Retail%20Sales%20in%202019V2.pdf.
|
| [2] |
Saikia, I.; Borah, A. J.; Phukan, P. Chem. Rev. 2016, 116, 6837.
pmid: 27199233 |
| [3] |
Alonso, F.; Beletskaya, I. P.; Yus, M. Chem. Rev. 2002, 102, 4009.
pmid: 12428984 |
| [4] |
Petrone, D. A.; Ye, J.; Lautens, M. Chem. Rev. 2016, 116, 8003.
pmid: 27341176 |
| [5] |
Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. Chem. Rev. 2019, 119, 2192.
pmid: 30480438 |
| [6] |
Teskey, C. J.; Lui, A. Y. W.; Greaney, M. F. Angew. Chem., Int. Ed. 2015, 54, 11677.
|
| [7] |
Cheng, H.-G.; Chen, S.; Chen, R.; Zhou, Q. Angew. Chem., Int. Ed. 2019, 58, 5832.
|
| [8] |
Gandeepan, P.; Koeller, J.; Korvorapun, K.; Mohr, J.; Ackermann, L. Angew. Chem., Int. Ed. 2019, 58, 9820.
|
| [9] |
Sagadevan, A.; Greaney, M. F. Angew. Chem., Int. Ed. 2019, 58, 9826.
doi: 10.1002/anie.v58.29 |
| [10] |
Xu, H.; Shang, M.; Dai, H.-X.; Yu, J.-Q. Org. Lett. 2015, 17, 3830.
pmid: 26204098 |
| [11] |
Yu, J.-Q.; Shi, Z. Topics in Current Chemistry, Vol. 292, Springer GmbH, Germany, 2010.
|
| [12] |
Latham, J.; Brandenburger, E.; Shepherd, S. A.; Menon, B. R. K.; Micklefield, J. Chem. Rev. 2018, 118, 232.
pmid: 28466644 |
| [13] |
Agarwal, V.; Miles, Z. D.; Winter, J. M.; Eustáquio, A. S.; El Gamal, A. A.; Moore, B. S. Chem. Rev. 2017, 117, 5619.
pmid: 28106994 |
| [14] |
Gribble, G. W. Progress in Chemistry of Organic Natural Products, Vol.91, Springer, New York, 2010.
|
| [15] |
Weichold, V.; Milbredt, D.; van Pée, K.-H. Angew. Chem. Int. Ed. 2016, 55, 6374.
doi: 10.1002/anie.201509573 |
| [16] |
Smith, D. R. M.; Grüschow, S.; Goss, R. J. M. Curr. Opin. Chem. Biol. 2013, 17, 276.
pmid: 23433955 |
| [17] |
Schallmey, A.; Schallmey, M. Appl. Microbiol. Biotechnol. 2016, 100, 7827.
pmid: 27502414 |
| [18] |
Leblanc, C.; Vilter, H.; Fournier, J. B.; Delage, L.; Potin, P.; Rebuffet, E.; Michel, G.; Solari, P. L.; Feiters, M. C.; Czjzek, M. Coord. Chem. Rev. 2015, 301-302, 134.
|
| [19] |
Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J. M. Org. Process Res. Dev. 2011, 15, 266.
|
| [20] |
Izumi, Y.; Xu, L.; di Tomaso, E.; Fukumura, D.; Jain, R. K. Nature 2002, 416, 279.
pmid: 11907566 |
| [21] |
Dong, C.-J.; Huang, F.-L.; Deng, H.; Schaffrath, C.; Spencer, J. B.; O'Hagan, D.; Naismith, J. H. Nature 2004, 427, 561.
pmid: 14765200 |
| [22] |
Hofrichter, M.; Ullrich, R. Appl. Microbiol. Biotechnol. 2006, 71, 276.
pmid: 16628447 |
| [23] |
Raugei, S.; Carloni, P. J. Phys. Chem. B 2006, 110, 3747.
pmid: 16494433 |
| [24] |
Noyori, R.; Aoki, M.; Sato, K. Chem. Commun. 2003,1977.
|
| [25] |
Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H. Science 2019, 366, 226.
pmid: 31601767 |
| [26] |
Morris, D. R.; Hager, L. P. J. Biol. Chem. 1966, 241, 1763.
pmid: 5949836 |
| [27] |
Ullrich, R.; Nüske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Appl. Environ. Microbiol. 2004, 70, 4575.
pmid: 15294788 |
| [28] |
Wever, R.; van der Horst, M. A. Dalton Trans. 2013, 42, 11778.
doi: 10.1039/c3dt50525a pmid: 23657250 |
| [29] |
Wever, R.; Krenn, B. E.; Renirie, R.In Methods in Enzymology, Vol. 605, Ed.: Moore, B. S., Academic Press, United States, 2018, p. 141.
|
| [30] |
Fernandez-Fueyo, E.; van Wingerden, M.; Renirie, R.; Wever, R.; Ni, Y.; Holtmann, D.; Hollmann, F. ChemCatChem 2015, 7, 4035.
|
| [31] |
Yu, Y.; Jin, Y.; Wu, P.-C.; Zhang, W. Chin. J. Catal. 2007, 28, 915. (in Chinese)
|
|
(于瑶, 靳艳, 吴佩春, 张卫, 催化学报, 2007, 28, 915.)
|
|
| [32] |
Zhang, B.-M.; Cao, X.-P.; Xue, S.; Xiao, T.-H.; Zhang, W. Chin. J. Catal. 2010, 31, 1293. (in Chinese)
|
|
(章表明, 曹旭鹏, 薛松, 肖通虎, 张卫, 催化学报, 2010, 31, 1293.)
|
|
| [33] |
Wischang, D.; Hartung, J. Tetrahedron 2012, 68, 9456.
|
| [34] |
Getrey, L.; Krieg, T.; Hollmann, F.; Schrader, J.; Holtmann, D. Green Chem. 2014, 16, 1104.
|
| [35] |
Kaur, R.; Darokar, M. P.; Chattopadhyay, S. K.; Krishna, V.; Ahmad, A. Med. Chem. Res. 2014, 23, 2212.
|
| [36] |
Frank, A.; Seel, C. J.; Groll, M.; Gulder, T. ChemBioChem 2016, 17, 2028.
doi: 10.1002/cbic.v17.21 |
| [37] |
Seel, C. J.; Králík, A.; Hacker, M.; Frank, A.; König, B.; Gulder, T. ChemCatChem 2018, 10, 3960.
|
| [38] |
Carter-Franklin, J. N.; Butler, A. J. Am. Chem. Soc. 2004, 126, 15060.
doi: 10.1021/ja047925p pmid: 15548002 |
| [39] |
Dong, J. J.; Fernandez-Fueyo, E.; Li, J.; Guo, Z.; Renirie, R.; Wever, R.; Hollmann, F. Chem. Commun. 2017.
|
| [40] |
Younes, S. H. H.; Tieves, F.; Lan, D.; Wang, Y.; Suess, P.; Brundiek, H.; Wever, R.; Hollmann, F. ChemSusChem 2020, 13, 1.
|
| [41] |
Tieves, F.; Willot, S. J.; van Schie, M.; Rauch, M. C. R.; Younes, S. H. H.; Zhang, W.; Dong, J.; Gomez de Santos, P.; Robbins, J. M.; Bommarius, B.; Alcalde, M.; Bommarius, A. S.; Hollmann, F. Angew. Chem., Int. Ed. 2019, 58, 7873.
|
| [42] |
Höfler, G. T.; But, A.; Younes, S. H. H.; Wever, R.; Paul, C. E.; Arends, I. W. C. E.; Hollmann, F. ACS Sustainable Chem. Eng. 2020, 8, 2602.
pmid: 32117647 |
| [43] |
Sandy, M.; Carter-Franklin, J. N.; Martin, J. D.; Butler, A. Chem. Commun. 2011, 47, 12086.
doi: 10.1039/c1cc15605e |
| [44] |
Sheldon, R. A. Green Chem. 2017, 19, 18.
|
| [45] |
But, A.; van Noord, A.; Poletto, F.; Sanders, J. P. M.; Franssen, M. C. R.; Scott, E. L. Mol. Catal. 2017, 443, 92.
|
| [46] |
But, A.; Le Nôtre, J.; Scott, E. L.; Wever, R.; Sanders, J. P. M. ChemSusChem 2012, 5, 1199.
doi: 10.1002/cssc.201200098 pmid: 22556065 |
| [47] |
Xu, X.; But, A.; Wever, R.; Hollmann, F. ChemCatChem 2020, 12, 2180.
|
| [48] |
Li, Y.; Ma, Y.; Li, P.; Zhang, X.; Ribitsch, D.; Alcalde, M.; Hollmann, F.; Wang, Y. ChemPlusChem 2020, 85, 254.
doi: 10.1002/cplu.201900751 pmid: 31951316 |
| [49] |
Bassanini, I.; Ferrandi, E. E.; Vanoni, M.; Ottolina, G.; Riva, S.; Crotti, M.; Brenna, E.; Monti, D. Eur. J. Org. Chem. 2017, 2017, 7186.
doi: 10.1002/ejoc.201701390 |
| [50] |
Thiel, D.; Doknić, D.; Deska, J. Nat. Commun. 2014, 5, 5278.
pmid: 25335580 |
| [51] |
Maurya, M. R.; Uprety, B.; Avecilla, F.; Adão, P.; Costa Pessoa, J. Dalton Trans. 2015, 44, 17736.
pmid: 26399883 |
| [52] |
Andersson, M.; Conte, V.; Di Furia, F.; Moro, S. Tetrahedron Lett. 1995, 36, 2675.
|
| [53] |
Conte, V.; Floris, B.; Galloni, P.; Silvagni, A. Pure Appl. Chem. 2005, 77, 1575.
|
| [54] |
Narender, M.; Reddy, M. S.; Nageswar, Y. V. D.; Rao, K. R. J. Mol. Catal. A 2006, 258, 10.
|
| [55] |
Claes, L.; Matthessen, R.; Rombouts, I.; Stassen, I.; De Baerdemaeker, T.; Depla, D.; Delcour, J. A.; Lagrain, B.; De Vos, D. E. ChemSusChem 2015, 8, 345.
pmid: 25470619 |
| [56] |
Claes, L.; Verduyckt, J.; Stassen, I.; Lagrain, B.; De Vos, D. E. Chem. Commun. 2015, 51, 6528.
|
| [57] |
Kaysser, L.; Bernhardt, P.; Nam, S.-J.; Loesgen, S.; Ruby, J. G.; Skewes-Cox, P.; Jensen, P. R.; Fenical, W.; Moore, B. S. J. Am. Chem. Soc. 2012, 134, 11988. 010967d1-e0a4-476e-9995-7ef92ef47f76
doi: 10.1021/ja305665f pmid: 22784372 |
| [58] |
Miles, Z. D.; Diethelm, S.; Pepper, H. P.; Huang, D. M.; George, J. H.; Moore, B. S. Nat. Chem. 2017, 9, 1235.
doi: 10.1038/nchem.2829 pmid: 29168495 |
| [59] |
Arnold, F. H. Angew. Chem., Int. Ed. 2018, 57, 4143.
|
| [60] |
Fasan, R.; Jennifer Kan, S. B.; Zhao, H. ACS Catal. 2019, 9, 9775.
pmid: 32728486 |
| [61] |
Qu, G.; Zhu, T.; Jiang, Y.-Y.; Wu, B.; Sun, Z.-T. Chin. J. Biotechnol. 2019, 35, 1843. (in Chinese)
|
|
(曲戈, 朱彤, 蒋迎迎, 吴边, 孙周通, 生物工程学报, 2019, 35, 1843.)
|
|
| [62] |
Freakley, S. J.; Kochius, S.; van Marwijk, J.; Fenner, C.; Lewis, R. J.; Baldenius, K.; Marais, S. S.; Opperman, D. J.; Harrison, S. T. L.; Alcalde, M.; Smit, M. S.; Hutchings, G. J. Nat. Commun. 2019, 10, 4178.
pmid: 31519878 |
| [63] |
van Schie, M. M. C. H.; Zhang,, W.; Tieves,, F.; Choi,, D. S.; Park,, C. B.; Burek,, B. O.; Bloh,, J. Z.; Arends,, I. W. C. E.; Paul,, C. E.; Alcalde,, M.; Hollmann,, F. ACS Catal. 2019, 9, 7409.
|
| [64] |
Zhang, W.; Fernández-Fueyo, E.; Ni, Y.; van Schie, M.; Gacs, J.; Renirie, R.; Wever, R.; Mutti, F. G.; Rother, D.; Alcalde, M.; Hollmann, F. Nat. Catal. 2018, 1, 55.
pmid: 29430568 |
| [65] |
Zhang, W.; Burek, B. O.; Fernandez-Fueyo, E.; Alcalde, M.; Bloh, J. Z.; Hollmann, F. Angew. Chem., Int. Ed. 2017, 56, 15451.
|
| [66] |
Ni, Y.; Fernández-Fueyo, E.; Baraibar, A. G.; Ullrich, R.; Hofrichter, M.; Yanase, H.; Alcalde, M.; van Berkel, W. J. H.; Hollmann, F. Angew. Chem. Int. Ed. 2016, 55, 798.
|
| [67] |
Willot, S. J. P.; Fernández-Fueyo, E.; Tieves, F.; Pesic, M.; Alcalde, M.; Arends, I. W. C. E.; Park, C. B.; Hollmann, F. ACS Catal. 2019, 9, 890.
doi: 10.1021/acscatal.8b03752 pmid: 30775065 |
| [68] |
Bormann, S.; van Schie, M. M. C. H.; De Almeida, T. P.; Zhang, W.; Stöckl, M.; Ulber, R.; Hollmann, F.; Holtmann, D. ChemSus- Chem 2019, 12, 4759.
|
| [69] |
Yuan, B.; Mahor, D.; Fei, Q.; Wever, R.; Alcalde, M.; Zhang, W.; Hollmann, F. ACS Catal. 2020, 10, 8277.
pmid: 32802571 |
| [70] |
Fernández-Fueyo, E.; Ni, Y.; Gomez Baraibar, A.; Alcalde, M.; van Langen, L. M.; Hollmann, F. J. Mol. Catal. B: Enzym. 2016, 134, 347.
|
| [1] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [2] | Jing Huang, Yihua Yang, Zhanhui Zhang, Shouxin Liu. Recent Progress on Green Methods and Technologies for Efficient Formation of Amide Bonds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 409-420. |
| [3] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [4] | Yixin Jiang, Boxiao Tang, Haibo Mao, Xuexia Chen, Yangjie Yu, Cuiying Quan, Zhaoyang Xu, Jinhui Shi, Yilin Liu. A Green, Recyclable and Carrier-Free Study for the Coupling Reaction of Alkenes with Aryl Iodides in H2O-Polyethylene Glycol (PEG-200) [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3210-3215. |
| [5] | Ran Zhou, Chunmei Yuan, Tao Zhang, Piao Mao, Yi Liu, Kaini Meng, Hui Xin, Wei Xue. Design, Synthesis and Bioactivity of Chalcone Derivative Containing Quinazolinone [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3196-3209. |
| [6] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| [7] | Kai Lu, Haoqi Qu, Xi Chen, Hui Qiu, Jing Zheng, Mengtao Ma. Catalyst-Free and Solvent-Free Hydroboration of Alkynes and Alkenes with Catecholborane [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2197-2205. |
| [8] | Guangli Xu, Jing Xu, Haidong Xu, Xiang Cui, Xingzhong Shu. Research Progress of Transition Metal Catalyzed Synthesis of 1,3- Conjugated Diene Compounds from Alkenes and Alkynes [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1899-1933. |
| [9] | Qian Dou, Taimin Wang, Lijing Fang, Hongbin Zhai, Bin Cheng. Recent Development of Photoinduced Iron-Catalysis in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1386-1415. |
| [10] | Shiquan Gao, Chuangjun Liu, Junfeng Yang, Junliang Zhang. Cobalt-Catalyzed Electrochemical Reductive Coupling of Alkynes and Alkenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1559-1565. |
| [11] | Linsheng Bai, Peng Hong, Anguo Ying. Research Progress of Functional Polyacrylonitrile Fiber in Promoting Organic Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1241-1270. |
| [12] | Baichuan Mo, Chunxia Chen, Jinsong Peng. Research Progress in Application of Lignin and Its Derivatives Supported Metal Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1215-1240. |
| [13] | Biao Ma, Miaomiao Zhang, Zhanyu Li, Jinsong Peng, Chunxia Chen. Recent Advance of Transition Metal-Free Catalyzed Suzuki-Type Cross Coupling Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 455-470. |
| [14] | Qiyang Li, Haiyan Zhang, Wenbo Liu. Research Progress in Transition-Metal-Free C—Si Bond Formation [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3470-3490. |
| [15] | Silin Chen, Yunhui Yang, Chao Chen, Congyang Wang. Advances in Transition-Metal-Catalyzed Keto Carbonyl-Directed C—H Bond Functionalization Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 1-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||