Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (3): 1136-1145.DOI: 10.6023/cjoc202211013 Previous Articles Next Articles
Special Issue: 有机氟化学虚拟合辑; 中国女科学家专辑
ARTICLES
收稿日期:2022-11-10
修回日期:2023-01-10
发布日期:2023-01-18
通讯作者:
张霄
作者简介:基金资助:
Rui Wanga, Lang Gaoa, Cen Zhoub, Xiao Zhanga( )
)
Received:2022-11-10
Revised:2023-01-10
Published:2023-01-18
Contact:
Xiao Zhang
About author:Supported by:Share
Rui Wang, Lang Gao, Cen Zhou, Xiao Zhang. Haloperfluoroalkylation of Unactivated Terminal Alkenes over Phenylphenothiazine-Based Porous Organic Polymers[J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1136-1145.
| Entry | Catalyst | Base | Solvent | Yieldb/% of 3a |
|---|---|---|---|---|
| 1 | PTH-POP1 | K2HPO4 | CH3CN | 96 (88)c |
| 2 | PTH-POP2 | K2HPO4 | CH3CN | 95 |
| 3 | PTH-POP1 | Na2HPO4 | CH3CN | 94 |
| 4 | PTH-POP1 | NaHCO3 | CH3CN | 57 |
| 5 | PTH-POP1 | Et3N | CH3CN | 47 |
| 6 | PTH-POP1 | DBU | CH3CN | 22 |
| 7 | PTH-POP1 | — | CH3CN | 89 |
| 8 | PTH-POP1 | K2HPO4 | DMF | 37 |
| 9 | PTH-POP1 | K2HPO4 | Acetone | 79 |
| 10 | PTH-POP1 | K2HPO4 | THF | 39 |
| 11 | PTH-POP1 | K2HPO4 | DCE | 94 |
| 12d | — | K2HPO4 | CH3CN | 0 |
| 13e | PTH-POP1 | K2HPO4 | CH3CN | 0 |
| Entry | Catalyst | Base | Solvent | Yieldb/% of 3a |
|---|---|---|---|---|
| 1 | PTH-POP1 | K2HPO4 | CH3CN | 96 (88)c |
| 2 | PTH-POP2 | K2HPO4 | CH3CN | 95 |
| 3 | PTH-POP1 | Na2HPO4 | CH3CN | 94 |
| 4 | PTH-POP1 | NaHCO3 | CH3CN | 57 |
| 5 | PTH-POP1 | Et3N | CH3CN | 47 |
| 6 | PTH-POP1 | DBU | CH3CN | 22 |
| 7 | PTH-POP1 | — | CH3CN | 89 |
| 8 | PTH-POP1 | K2HPO4 | DMF | 37 |
| 9 | PTH-POP1 | K2HPO4 | Acetone | 79 |
| 10 | PTH-POP1 | K2HPO4 | THF | 39 |
| 11 | PTH-POP1 | K2HPO4 | DCE | 94 |
| 12d | — | K2HPO4 | CH3CN | 0 |
| 13e | PTH-POP1 | K2HPO4 | CH3CN | 0 |
| Entry | Catalyst | Reductant | Solvent | Yieldb/% of 5a |
|---|---|---|---|---|
| 1 | PTH-POP1 | Sodium ascorbate | CH3CN/CH3OH | 99 (86)c |
| 2 | PTH-POP2 | Sodium ascorbate | CH3CN/CH3OH | 97 |
| 3 | PTH-POP1 | DIPEA | CH3CN/CH3OH | 79 |
| 4 | PTH-POP1 | Et3N | CH3CN/CH3OH | 96 |
| 5d | PTH-POP1 | — | CH3CN/CH3OH | 5 |
| 6e | — | Sodium ascorbate | CH3CN/CH3OH | 2 |
| 7f | PTH-POP1 | Sodium ascorbate | CH3CN/CH3OH | 0 |
| Entry | Catalyst | Reductant | Solvent | Yieldb/% of 5a |
|---|---|---|---|---|
| 1 | PTH-POP1 | Sodium ascorbate | CH3CN/CH3OH | 99 (86)c |
| 2 | PTH-POP2 | Sodium ascorbate | CH3CN/CH3OH | 97 |
| 3 | PTH-POP1 | DIPEA | CH3CN/CH3OH | 79 |
| 4 | PTH-POP1 | Et3N | CH3CN/CH3OH | 96 |
| 5d | PTH-POP1 | — | CH3CN/CH3OH | 5 |
| 6e | — | Sodium ascorbate | CH3CN/CH3OH | 2 |
| 7f | PTH-POP1 | Sodium ascorbate | CH3CN/CH3OH | 0 |
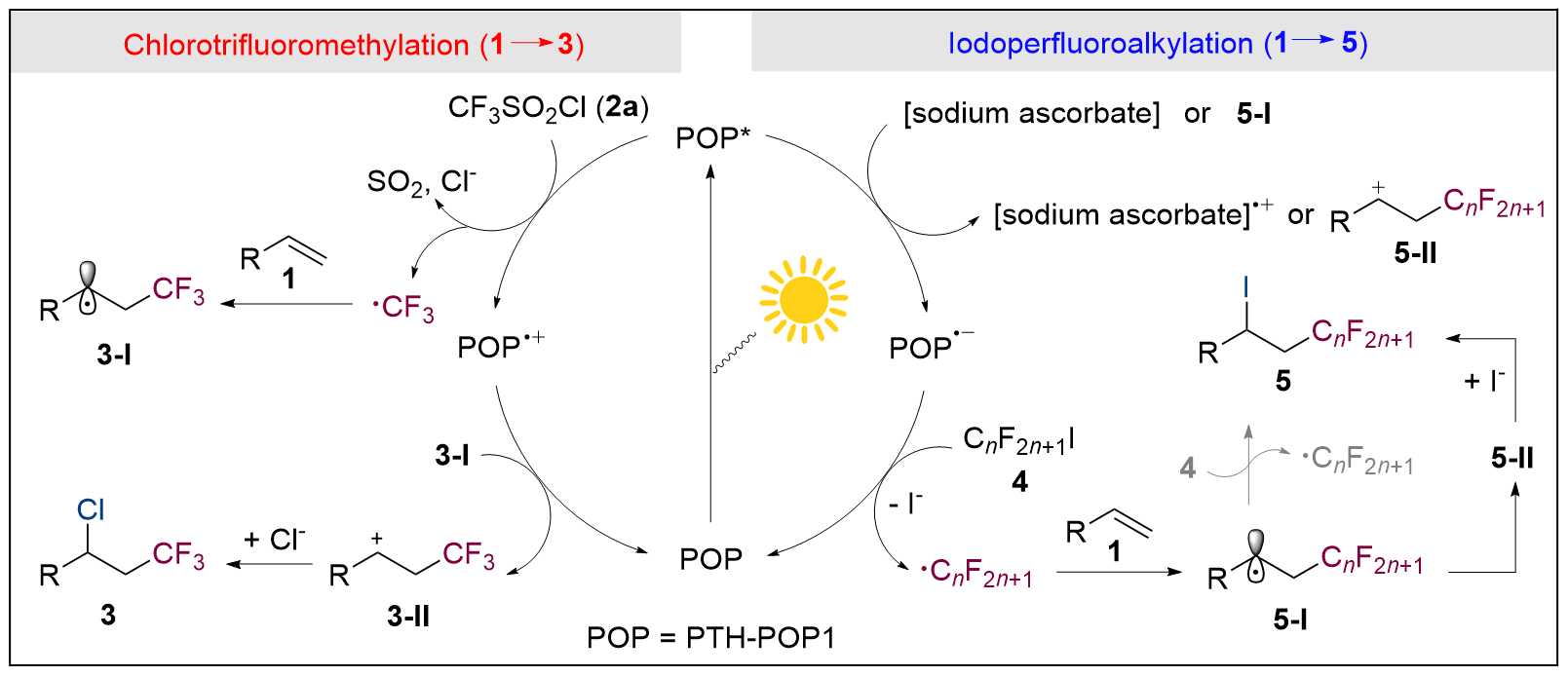
| [1] |
For selected reviews on visible-light photocatalysis, see: (a) Xuan, J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828.
doi: 10.1002/anie.201200223 pmid: 33881207 |
|
(b) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322.
doi: 10.1021/cr300503r pmid: 33881207 |
|
|
(c) Lang, X.; Chen, X.; Zhao, J. Chem. Soc. Rev. 2014, 43, 473.
doi: 10.1039/C3CS60188A pmid: 33881207 |
|
|
(d) Zeng, L.; Guo, X.; He, C.; Duan, C. ACS Catal. 2016, 6, 7935.
doi: 10.1021/acscatal.6b02228 pmid: 33881207 |
|
|
(e) Ravelli, D.; Protti, S.; Fagnoni, M. Chem. Rev. 2016, 116, 9850.
doi: 10.1021/acs.chemrev.5b00662 pmid: 33881207 |
|
|
(f) Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075.
doi: 10.1021/acs.chemrev.6b00057 pmid: 33881207 |
|
|
(g) Teixeira, I. F.; Barbosa, E. C. M.; Tsan, S. C. E.; Camargo, P. H. C. Chem. Soc. Rev. 2018, 47, 7783.
doi: 10.1039/c8cs00479j pmid: 33881207 |
|
|
(h) Marzo, L.; Pagire, S. K.; Reiser, O.; König, B. Angew. Chem., Int. Ed. 2018, 57, 10034.
doi: 10.1002/anie.v57.32 pmid: 33881207 |
|
|
(i) Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Sci. China: Chem. 2019, 62, 24.
doi: 10.1007/s11426-018-9399-2 pmid: 33881207 |
|
|
(j) Riente, P.; Noël, T. Catal. Sci. Technol. 2019, 9, 5186.
doi: 10.1039/c9cy01170f pmid: 33881207 |
|
|
(k) Li, J.-Y.; Li, Y.-H.; Qi, M.-Y.; Lin, Q.; Tang, Z.-R.; Xu, Y.-J. ACS Catal. 2020, 10, 6262.
doi: 10.1021/acscatal.0c01567 pmid: 33881207 |
|
|
(l) Wu, H.-L.; Li, X.-B.; Tung, C.-H.; Wu, L.-Z. Chem. Commun. 2020, 56, 15496.
doi: 10.1039/D0CC05870J pmid: 33881207 |
|
|
(m) Liu, J.; Fu, W.; Liao, Y.; Fan, J.; Xiang, Q. J. Mater. Sci. Technol. 2021, 91, 224.
doi: 10.1016/j.jmst.2021.03.017 pmid: 33881207 |
|
|
(n) Tlili, A.; Lakhdar, S. Angew. Chem., Int. Ed. 2021, 60, 19526.
doi: 10.1002/anie.202102262 pmid: 33881207 |
|
|
(o) Cheng, Y.-Z.; Feng, Z.; Zhang, X.; You, S.-L. Chem. Soc. Rev. 2022, 51, 2145.
doi: 10.1039/C9CS00311H pmid: 33881207 |
|
| [2] |
For selected reviews, see: (a) Wong, Y.-L.; Tobin, J. M.; Xu, Z.; Vilela, F. J. Mater. Chem. A 2016, 4, 18677.
doi: 10.1039/C6TA07697A |
|
(b) Li, R.; Byun, J.; Huang, W.; Ayed, C.; Wang, L.; Zhang, K. A. I. ACS Catal. 2018, 8, 4735.
doi: 10.1021/acscatal.8b00407 |
|
|
(c) Byun, J.; Zhang, K. A. I. Mater. Horiz. 2020, 7, 15.
doi: 10.1039/C9MH01071H |
|
|
(d) Zhang, T.; Xing, G.; Chen, W.; Chen, L. Mater. Chem. Front. 2020, 4, 332.
doi: 10.1039/C9QM00633H |
|
|
(e) Gisbertz, S.; Pieber, B. ChemPhotoChem 2020, 4, 456.
doi: 10.1002/cptc.v4.7 |
|
|
(f) Xu, Z.-Y.; Luo, Y.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. J. Org. Chem. 2020, 40, 3777. (in Chinese)
doi: 10.6023/cjoc202003070 |
|
|
(徐子悦, 罗驿, 王辉, 张丹维, 黎占亭, 有机化学, 2020, 40, 3777.)
doi: 10.6023/cjoc202003070 |
|
|
(g) Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Xu, P.; Wang, H.; Huang, D.; Liu, Y.; Shao, B.; Liang, Q.; Wang, D.; He, Q.; Qin, L.; Fu, Y. J. Mater. Chem. A 2020, 8, 6434.
doi: 10.1039/D0TA01102A |
|
|
(h) Wang, T.-X.; Liang, H.-P.; Anito, D. A.; Ding, X.; Han, B.-H. J. Mater. Chem. A 2020, 8, 7003.
doi: 10.1039/D0TA00364F |
|
|
(i) Xiao, J.; Liu, X.; Pan, L.; Shi, C.; Zhang, X.; Zou, J.-J. ACS Catal. 2020, 10, 12256.
doi: 10.1021/acscatal.0c03480 |
|
|
(j) Ferguson, C. T. J.; Zhang, K. A. I. ACS Catal. 2021, 11, 9547.
doi: 10.1021/acscatal.1c02056 |
|
|
(k) Ji, W.; Wang, T.-X.; Ding, X.; Lei, S.; Han, B.-H. Coord. Chem. Rev. 2021, 439, 213875.
doi: 10.1016/j.ccr.2021.213875 |
|
|
(l) Qian, Z.; Zhang, K. A. I. Sol. RRL. 2021, 5, 2000489.
doi: 10.1002/solr.v5.2 |
|
|
(m) Zhang, Z.; Jia, J.; Zhi, Y.; Ma, S.; Liu, X. Chem. Soc. Rev. 2022, 51, 2444.
doi: 10.1039/D1CS00808K |
|
|
For selected examples, see:
|
|
|
(n) Gui, Q.-W.; Teng, F.; Li, Z.-C.; Xiong, Z.-Y.; Jin, X.-F.; Lin, T.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1907.
doi: 10.1016/j.cclet.2021.01.021 |
|
|
(o) Wang, Z.; Liu, Q.; Liu, R.; Ji, Z.; Li, Y.; Zhao, X.; Wei, W. Chin. Chem. Lett. 2022, 33, 1479.
doi: 10.1016/j.cclet.2021.08.036 |
|
|
(p) Ma, C.-H.; Zhao, L.; He, X.; Jiang, Y.-Q.; Yu, B. Org. Chem. Front. 2022, 9, 1445.
doi: 10.1039/D1QO01870A |
|
| [3] |
For selected examples, see: (a) Liang, H.-P.; Chen, Q.; Han, B.-H. ACS Catal. 2018, 8, 5313.
doi: 10.1021/acscatal.7b04494 |
|
(b) Luo, J.; Lu, J.; Zhang, J. J. Mater. Chem. A 2018, 6, 15154.
doi: 10.1039/C8TA05329D |
|
|
(c) Zhi, Y.; Ma, S.; Xia, H.; Zhang, Y.; Shi, Z.; Mu, Y.; Liu, X. Appl. Catal. B 2019, 244, 36.
doi: 10.1016/j.apcatb.2018.11.032 |
|
|
(d) Zhang, W.; Li, S.; Tang, X.; Tang, J.; Pan, C.; Yu, G. Appl. Catal. B 2020, 272, 118982.
doi: 10.1016/j.apcatb.2020.118982 |
|
|
(e) Xu, Z.-Y.; Luo, Y.; Zhang, D.-W.; Wang, H.; Sun, X.-W.; Li, Z.-T. Green Chem. 2020, 22, 136.
doi: 10.1039/C9GC03688A |
|
|
(f) An, W.-K.; Zheng, S.-J.; Zhang, H.-X.; Shang, T.-T.; Wang, H.-R.; Xu, X.-J.; Jin, Q.; Qin, Y.; Ren, Y.; Jiang, S.; Xu, C.-L.; Hou, M.-S.; Pan, Z. Green Chem. 2021, 23, 1292.
doi: 10.1039/D0GC03719B |
|
|
(g) Liu, H.-K.; Lei, Y.-F.; Tian, P.-J.; Wang, H.; Zhao, X.; Li, Z.-T.; Zhang, D.-W. J. Mater. Chem. A 2021, 9, 6361.
doi: 10.1039/D0TA12267J |
|
|
(h) Guo, L.; Wang, X.; Zhan, Z.; Zhao, Y.; Chen, L.; Liu, T.; Tan, B.; Jin, S. Chem. Mater. 2021, 33, 1994.
doi: 10.1021/acs.chemmater.0c03716 |
|
|
(i) Li, S.; Zhang, W.; Yang, S.; Chen, F.; Pan, C.; Tang, J.; Zhang, K. A. I.; Yu, G. Chem. Eng. J. 2021, 408, 127261.
doi: 10.1016/j.cej.2020.127261 |
|
|
(j) Zhang, H.; Zhou, C.; Zheng, Y.; Zhang, X. Green Chem. 2021, 23, 8878.
doi: 10.1039/D1GC03165A |
|
|
(k) Fu, X.-Y.; Si, Y.-F.; Qiao, L.-P.; Zhao, Y.-F.; Chen, X.-L.; Yu, B. Adv. Synth. Catal. 2022, 364, 574.
doi: 10.1002/adsc.v364.3 |
|
|
(l) Zhu, S.-S.; Liu, Y.; Chen, X.-L.; Qu, L.-B.; Yu, B. ACS Catal. 2022, 12, 126.
doi: 10.1021/acscatal.1c03765 |
|
|
(m) Li, X.; Zhang, F.; Wang, Y.; Xiong, K.; Lang, X. J. Mater. Chem. A 2022, 10, 14965.
doi: 10.1039/D2TA02603A |
|
|
(n) Zhou, C.; Wang, R.; Gao, L.; Huang, X.; Zhang, X. ACS Appl. Mater. Interfaces 2022, 14, 30962.
doi: 10.1021/acsami.2c08766 |
|
|
(o) Lan, F.; Liu, C.-S.; Zhou, C.; Huang, X.; Wu, J.-Y.; Zhang, X. J. Mater. Chem. A 2022, 10, 16578.
doi: 10.1039/D2TA03341K |
|
|
(p) An, W.-K.; Zheng, S.-J.; Xu, X.; Liu, L.-J.; Ren, J.-S.; Fan, L.; Yang, Z.-K.; Ren, Y.; Xu, C. Appl. Catal. B 2022, 316, 121630.
doi: 10.1016/j.apcatb.2022.121630 |
|
|
(q) Zhu, S.-S.; Zuo, L.; Liu, Y.; Yu, B. Green Chem. 2022, 24, 8725.
doi: 10.1039/D2GC02950B |
|
|
(r) Shi, A.; Sun, K.; Wu, Y.; Xiang, P.; Krylov, I. B.; Terent’ev, A. O.; Chen, X.; Yu, B. J. Catal. 2022, 415, 28.
doi: 10.1016/j.jcat.2022.09.027 |
|
|
(s) Huang, X.; Liu, S.; Liu, G.; Tao, Y.; Wang, C.; Zhang, Y.; Li, Z.; Wang, H.; Zhou, Z.; Shen, G.; Xue, Z.; Sun, D. Appl. Catal. B 2023, 323, 122134.
doi: 10.1016/j.apcatb.2022.122134 |
|
| [4] |
(a) Muller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
doi: 10.1126/science.1131943 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C |
|
|
(c) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
doi: 10.1021/jm800219f |
|
|
(d) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
doi: 10.1021/cr4002879 |
|
| [5] |
For selected reviews, see: (a) Liu, H.; Gu, Z.; Jiang, X. Adv. Synth. Catal. 2013, 355, 617.
doi: 10.1002/adsc.201200764 pmid: 26464314 |
|
(b) Koike, T.; Akita, M. Top. Catal. 2014, 57, 967.
doi: 10.1007/s11244-014-0259-7 pmid: 26464314 |
|
|
(c) Zhang, C. Adv. Synth. Catal. 2014, 356, 2895.
doi: 10.1002/adsc.201400370 pmid: 26464314 |
|
|
(d) Barata-Vallejo, S.; Bonesi, S. M.; Postigo, A. Org. Biomol. Chem. 2015, 13, 11153.
doi: 10.1039/c5ob01486g pmid: 26464314 |
|
|
(e) Chatterjee, T.; Iqbal, N.; You, Y.; Cho, E. J. Acc. Chem. Res. 2016, 49, 2284.
doi: 10.1021/acs.accounts.6b00248 pmid: 26464314 |
|
|
For selected examples, see:
pmid: 26464314 |
|
|
(f) Liu, J.; Li, L.; Yu, L.; Tang, L.; Chen, Q.; Shi, M. Adv. Synth. Catal. 2018, 360, 2959.
doi: 10.1002/adsc.v360.15 pmid: 26464314 |
|
|
(g) Zhu, M.; Zhou, K.; Zhang, X.; You, S.-L. Org. Lett. 2018, 20, 4379.
doi: 10.1021/acs.orglett.8b01899 pmid: 26464314 |
|
|
(h) Wei, Z.; Qi, S.; Xu, Y.; Liu, H.; Wu, J.; Li, H.; Xia, C.; Duan, G. Adv. Synth. Catal. 2019, 361, 5490.
doi: 10.1002/adsc.v361.23 pmid: 26464314 |
|
|
(i) Guo, Y.-Q.; Wang, K.; Wang, R.; Song, H.; Liu, Y.; Wang, Q. Adv. Synth. Catal. 2021, 363, 1651.
doi: 10.1002/adsc.v363.6 pmid: 26464314 |
|
| [6] |
For a recent review, see: (a) Fu, B.; Escorihuela, J.; Han, J.; Fustero, S.; Barrio, P.; Sodeoka, M.; Kawamura, S.; Sorochinsky, A.; Soloshonok, V. A. Molecules 2021, 26, 7221.
doi: 10.3390/molecules26237221 pmid: 30793126 |
|
For selected examples, see: (b) Oh, S. H.; Malpani, Y. R.; Ha, N.; Jung, Y.-S.; Han, S. B. Org. Lett. 2014, 16, 1310.
doi: 10.1021/ol403716t pmid: 30793126 |
|
|
(c) Tang, X.-J.; Dolbier Jr, W. R. Angew. Chem., Int. Ed. 2015, 54, 4246.
doi: 10.1002/anie.201412199 pmid: 30793126 |
|
|
(d) Bagal, D. B.; Kachkovskyi, G.; Knorn, M.; Rawner, T.; Bhanage, B. M.; Reiser, O. Angew. Chem., Int. Ed. 2015, 54, 6999.
doi: 10.1002/anie.201501880 pmid: 30793126 |
|
|
(e) Alkan-Zambada, M.; Hu, X. Organometallics 2018, 37, 3928.
doi: 10.1021/acs.organomet.8b00585 pmid: 30793126 |
|
|
(f) Zhang, W.; Lin, J.-H.; Xiao, J.-C. J. Fluorine Chem. 2018, 215, 25.
doi: 10.1016/j.jfluchem.2018.09.001 pmid: 30793126 |
|
|
(g) Nicholls, T. P.; Caporale, C.; Massi, M.; Gardiner, M. G.; Bissember, A. C. Dalton Trans. 2019, 48, 7290.
doi: 10.1039/c8dt04116d pmid: 30793126 |
|
|
(h) Maeda, K.; Kurahashi, T.; Matsubara, S. Eur. J. Org. Chem. 2019, 2019, 4613.
doi: 10.1002/ejoc.201900834 pmid: 30793126 |
|
|
(i) Engl, S.; Reiser, O. Eur. J. Org. Chem. 2020, 2020, 1523.
doi: 10.1002/ejoc.v2020.10 pmid: 30793126 |
|
|
(j) Wu, D.; Fan, W.; Wu, L.; Chen, P.; Liu, G. ACS Catal. 2022, 12, 5284.
doi: 10.1021/acscatal.2c00623 pmid: 30793126 |
|
| [7] |
Quan, Y.; Shi, W.; Song, Y.; Jiang, X.; Wang, C.; Lin, W. J. Am. Chem. Soc. 2021, 143, 3075.
doi: 10.1021/jacs.1c01083 |
| [8] |
Shi, Y.; Zhang, T.; Jiang, X.-M.; Xu, G.; He, C.; Duan, C. Nat. Commun. 2020, 11, 5384.
doi: 10.1038/s41467-020-19172-3 |
| [9] |
For selected examples, see: (a) Treat, N. J.; Sprafke, H.; Kramer, J. W.; Clark, P. G.; Barton, B. E.; Alaniz, J. R. D.; Fors, B. P.; Hawker, C. J. J. Am. Chem. Soc. 2014, 136, 16096.
doi: 10.1021/ja510389m |
|
(b) Wang, H.; Jui, N. T. J. Am. Chem. Soc. 2018, 140, 163.
doi: 10.1021/jacs.7b12590 |
|
|
(c) Speck, F.; Rombach, D.; Wagenknecht, H.-A. Beilstein J. Org. Chem. 2019, 15, 52.
doi: 10.3762/bjoc.15.5 |
|
|
(d) Lu, F.-D.; Liu, D.; Zhu, L.; Lu, L.-Q.; Yang, Q.; Zhou, Q.-Q.; Wei, Y.; Lan, Y.; Xiao, W.-J. J. Am. Chem. Soc. 2019, 141, 6167.
doi: 10.1021/jacs.9b02338 |
|
|
(e) Wang, R.; Zhou, C.; Huang, X.; Wu, J.-Y.; Zhang, X. ACS Sustainable Chem. Eng. 2022, 10, 4650.
doi: 10.1021/acssuschemeng.2c00054 |
|
| [10] |
For selected reviews, see: (a) Bag, D.; Kour, H.; Sawant, S. D. Org. Biomol. Chem. 2020, 18, 8278.
doi: 10.1039/D0OB01454K |
|
(b) Liu, T.; Liu, J.; He, J.; Hong, Y.; Zhou, H.; Liu, Y.-L.; Tang, S. Synthesis 2022, 54, 1919.
doi: 10.1055/s-0040-1719900 |
|
|
For selected examples, see: (c) Wallentin, C.-J.; Nguyen, J. D.; Finkbeiner, P.; Stephenson, C. R. J. J. Am. Chem. Soc. 2012, 134, 8875.
doi: 10.1021/ja300798k |
|
|
(d) Mao, L.-L.; Cong, H. ChemSusChem 2017, 10, 4461.
doi: 10.1002/cssc.201701382 |
|
|
(e) Rawner, T.; Lutsker, E.; Kaiser, C. A.; Reiser, O. ACS Catal. 2018, 8, 3950.
doi: 10.1021/acscatal.8b00847 |
|
|
(f) Zhang, T.; Wang, P.; Gao, Z.; An, Y.; He, C.; Duan, C. RSC Adv. 2018, 8, 32610.
doi: 10.1039/C8RA06181E |
|
|
(g) Filippini, G.; Longobardo, F.; Forster, L.; Criado, A.; Di Carmine, G.; Nasi, L.; D’Agostino, C.; Melchionna, M.; Fornasiero, P.; Prato, M. Sci. Adv. 2020, 6, eabc9923.
doi: 10.1126/sciadv.abc9923 |
|
|
(h) Liu, Z.; Li, C.; Chen, J.; Li, X.; Luo, F.; Cheng, F.; Liu, J.-J. Inorg. Chem. Front. 2022, 9, 111.
doi: 10.1039/D1QI01206A |
|
| [11] |
Nagib, D. A.; MacMillan, D. W. C. Nature 2011, 480, 224.
doi: 10.1038/nature10647 |
| [12] |
Cismesia, M. A.; Yoon, T. P. Chem. Sci. 2015, 6, 5426.
pmid: 26668708 |
| [13] |
Li, Z.; Wang, J.-A.; Ma, S.; Zhang, Z.; Zhi, Y.; Zhang, F.; Xia, H.; Henkelman, G.; Liu, X. Appl. Catal. B 2022, 310, 121335.
doi: 10.1016/j.apcatb.2022.121335 |
| [14] |
Beniazza, R.; Atkinson, R.; Absalon, C.; Castet, F.; Denisov, S. A.; McClenaghan, N. D.; Lastécouères, D.; Vincent, J. M. Adv. Synth. Catal. 2016, 358, 2949.
doi: 10.1002/adsc.v358.18 |
| [1] | Jiyu Liu, Shengyu Li, Kuan Chen, Yin Zhu, Yuan Zhang. Triphenylamine-Based Ordered Mesoporous Polymer as a Metal-Free Photocatalyst for Oxidation of Thiols to Disulfide [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 605-612. |
| [2] | Jianghu Dong, Liangming Xuan, Chi Wang, Chenxi Zhao, Haifeng Wang, Qiongjiao Yan, Wei Wang, Fen'er Chen. Recent Advances in Visible-Light-Induced C(3)—H Functionalization of Quinoxalinones under Transition-Metal-Free or Photocatalyst-Free [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 111-136. |
| [3] | Xu Liao, Zeyu Wang, Wufei Tang, Jinqing Lin. Progress in Porous Organic Polymer for Chemical Fixation of Carnbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2699-2710. |
| [4] | Shiguo Ou, Ruirui Chai, Jiahao Li, Dawei Wang, Xinxin Sang. Metal-Organic Framework Derived Phytate-Iron for Efficient Synthesis of 2-Arylbenzoxazole via Hydrogen Transfer Strategy [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2934-2945. |
| [5] | Yijun Shi, Xinyue Sun, Han Cao, Fusheng Bie, Jie Ma, Zhe Liu, Xingshun Cong. Thioesterification of Esters with Primary Aliphatic Thiols at Room Temperature [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2499-2505. |
| [6] | Baichuan Mo, Chunxia Chen, Jinsong Peng. Research Progress in Application of Lignin and Its Derivatives Supported Metal Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1215-1240. |
| [7] | Qiyang Li, Haiyan Zhang, Wenbo Liu. Research Progress in Transition-Metal-Free C—Si Bond Formation [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3470-3490. |
| [8] | Rui Bai, Xujuan Liu, Wenyu Luo, Shanshan Liu, Linyu Jiao. Research Progress of Chan-Lam Coupling Reaction in Heterogeneous Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2342-2354. |
| [9] | JIan Xiao, Zhiying Wu, Ziyi Chen, Pengfei Zhao. Tetraethylenepentamine Functionalized Phenolic Resin as Highly Active Acid-Base Bifunctional Catalyst for Knoevenagel Condensation Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1179-1187. |
| [10] | Qiang Huang, Tingting Deng, Jiayun Zhu, Jun Li, Feifei Li. Study on the Green Synthesis of β-Hydroxy-1,2,3-triazoles Catalyzed by An Amino-Functionalized Graphene-Supported Ag-Cu Composites [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 534-542. |
| [11] | Anguo Ying, Linsheng Bai, Hailiang Hou, Songlin Xu, Xiaotong Lu, Limin Wang. Research on Thia-Michael Addition Tandem Reactions Catalyzed by AlCl3@MNPs [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3843-3852. |
| [12] | Yaoyao Zhang, Lijie Zhou, Biao Han, Weishuang Li, Bojie Li, Lei Zhu. Research Progress of Chitosan Supported Copper Catalyst in Organic Reactions [J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 33-53. |
| [13] | Xin Chen, Chunxia Chen, Jinsong Peng. Research Progress of Cellulose and Its Derivatives Supported Copper Catalyst Catalyzed Organic Reactions [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1319-1336. |
| [14] | Hanlin Deng, Xiansheng Luo, Zhihua Li, Jiangying Zhao, Muhua Huang. Synthesis of Novel Porous Organic Materials Based on Phloroglucinol and Its Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 624-641. |
| [15] | Yuxuan Chen, Qi Chen, Zhanhui Zhang. Application of Covalent Organic Framework Materials as Heterogeneous Ligands in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 3826-3843. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||