有机化学 ›› 2021, Vol. 41 ›› Issue (5): 1789-1803.DOI: 10.6023/cjoc202011005 上一篇 下一篇
综述与进展
孙忠文1,*( ), 张聪聪1, 陈丽君1, 谢惠定1, 柳波1, 刘丹丹1,*(
), 张聪聪1, 陈丽君1, 谢惠定1, 柳波1, 刘丹丹1,*( )
)
收稿日期:2020-11-04
修回日期:2020-12-10
发布日期:2020-12-31
通讯作者:
孙忠文, 刘丹丹
基金资助:
Zhongwen Sun1,*( ), Congcong Zhang1, Lijun Chen1, Huiding Xie1, Bo Liu1, Dandan Liu1,*(
), Congcong Zhang1, Lijun Chen1, Huiding Xie1, Bo Liu1, Dandan Liu1,*( )
)
Received:2020-11-04
Revised:2020-12-10
Published:2020-12-31
Contact:
Zhongwen Sun, Dandan Liu
About author:Supported by:文章分享
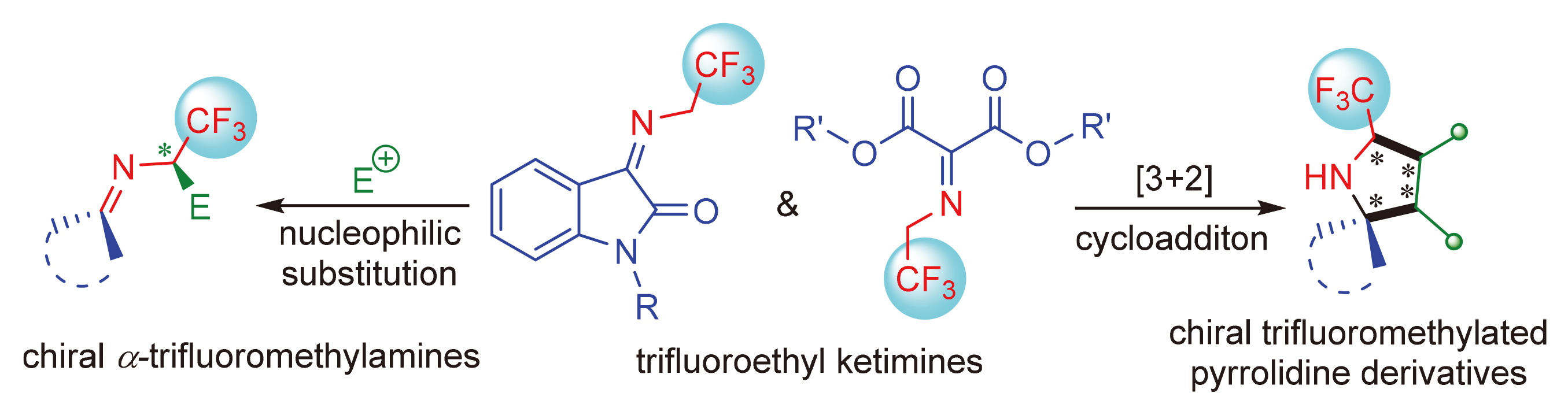
氟原子或含氟基团在材料科学、药物化学等领域的生物活性分子中广泛存在. 三氟乙基酮亚胺同时存在高活性亲电和亲核中心, 是良好的1,3-偶极子. 因其在催化不对称构建含有三氟甲基立体中心的反应中具有极高的研究价值而备受关注. 以三氟乙基酮亚胺的底物和反应类型为主线, 综述了近五年三氟乙基酮亚胺参与的催化不对称反应研究进展, 同时对该领域的未来发展进行了展望.
孙忠文, 张聪聪, 陈丽君, 谢惠定, 柳波, 刘丹丹. 三氟乙基酮亚胺参与的催化不对称反应研究进展[J]. 有机化学, 2021, 41(5): 1789-1803.
Zhongwen Sun, Congcong Zhang, Lijun Chen, Huiding Xie, Bo Liu, Dandan Liu. Recent Advances in Catalytic Asymmetric Reactions Involving Trifluoroethyl Ketimines[J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 1789-1803.
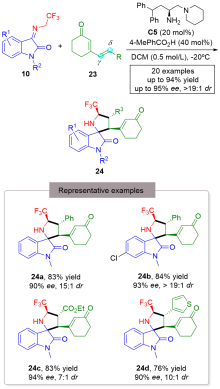
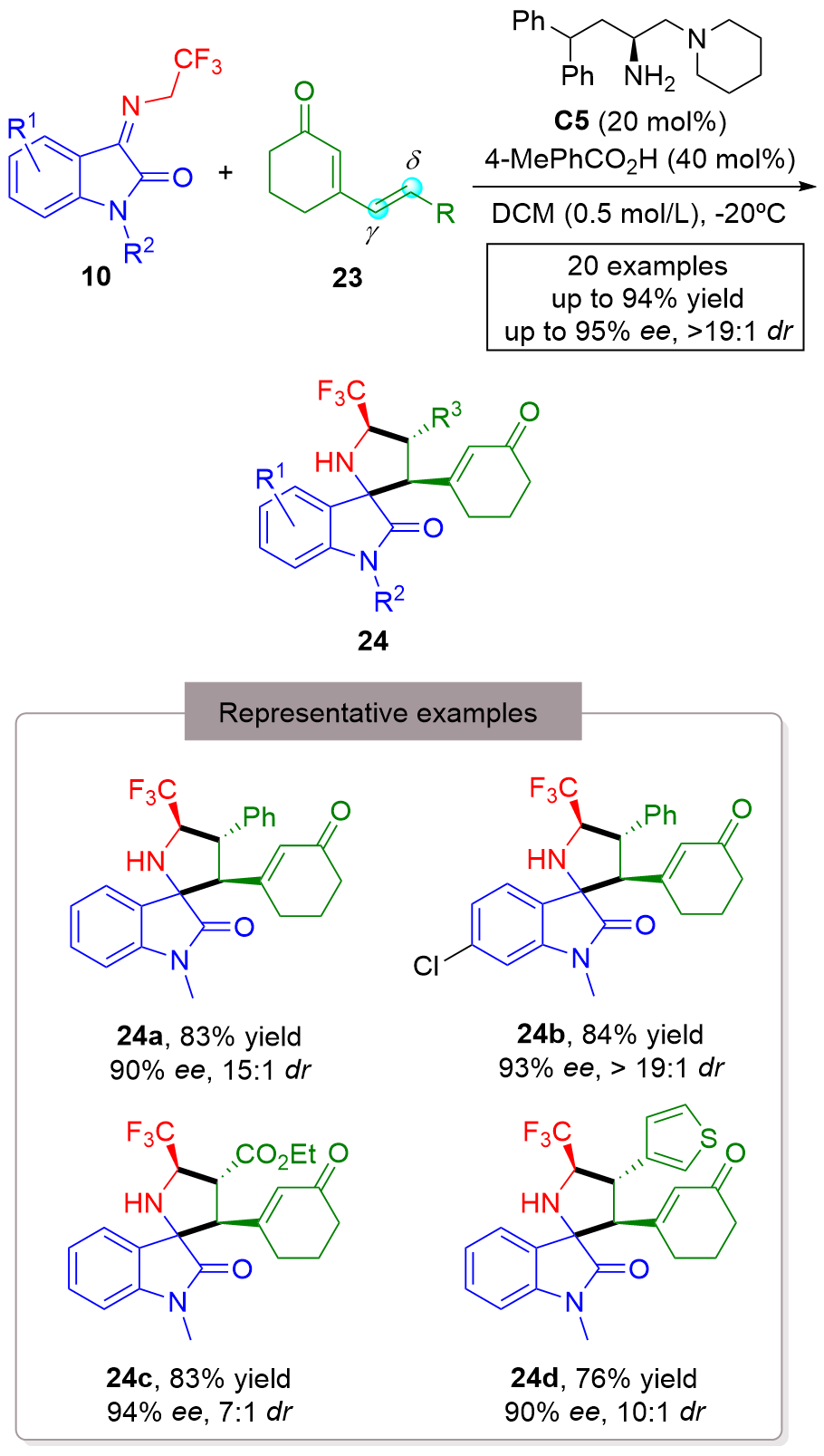
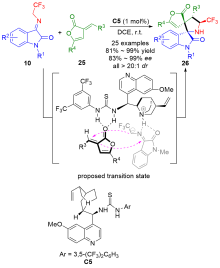
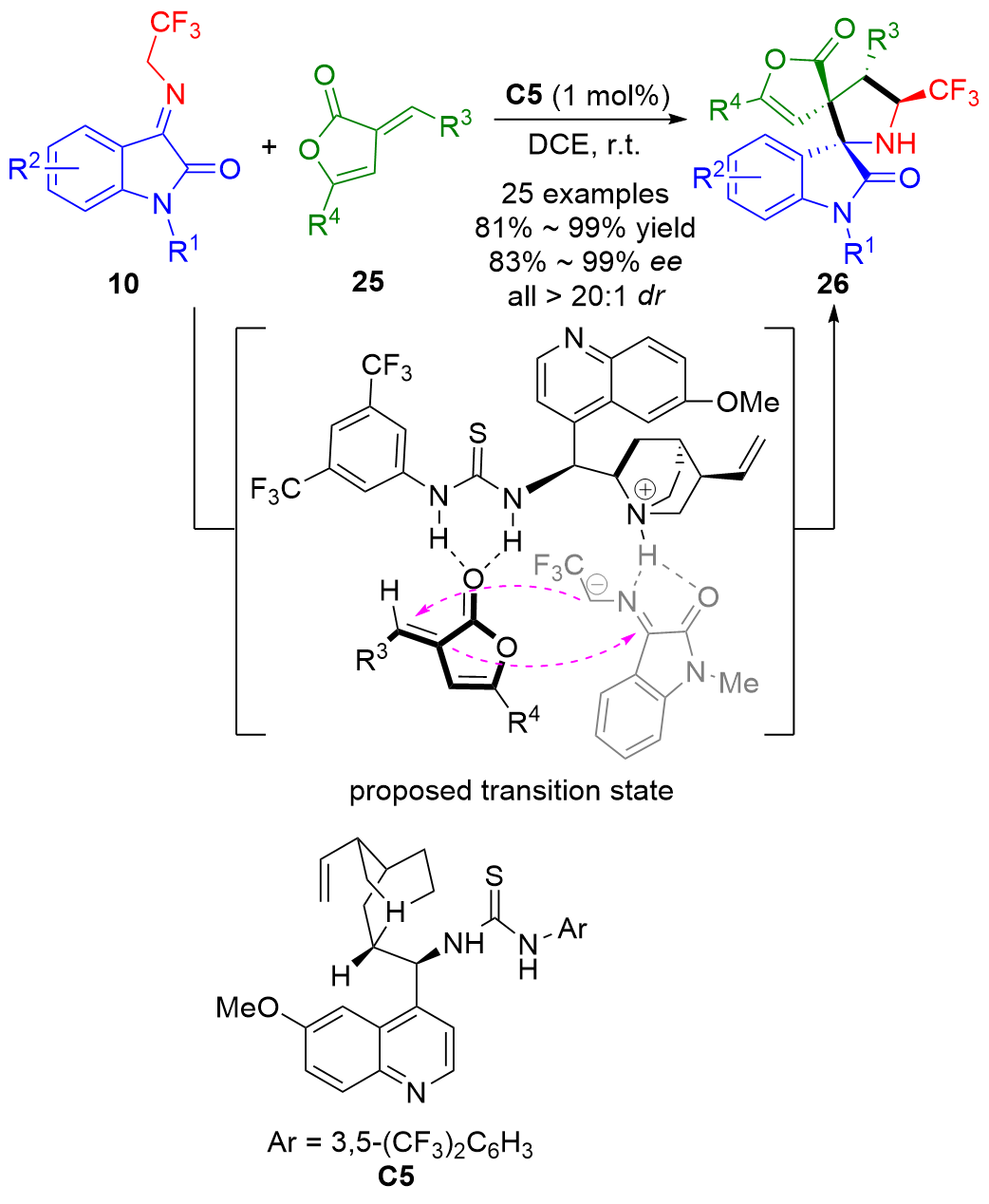

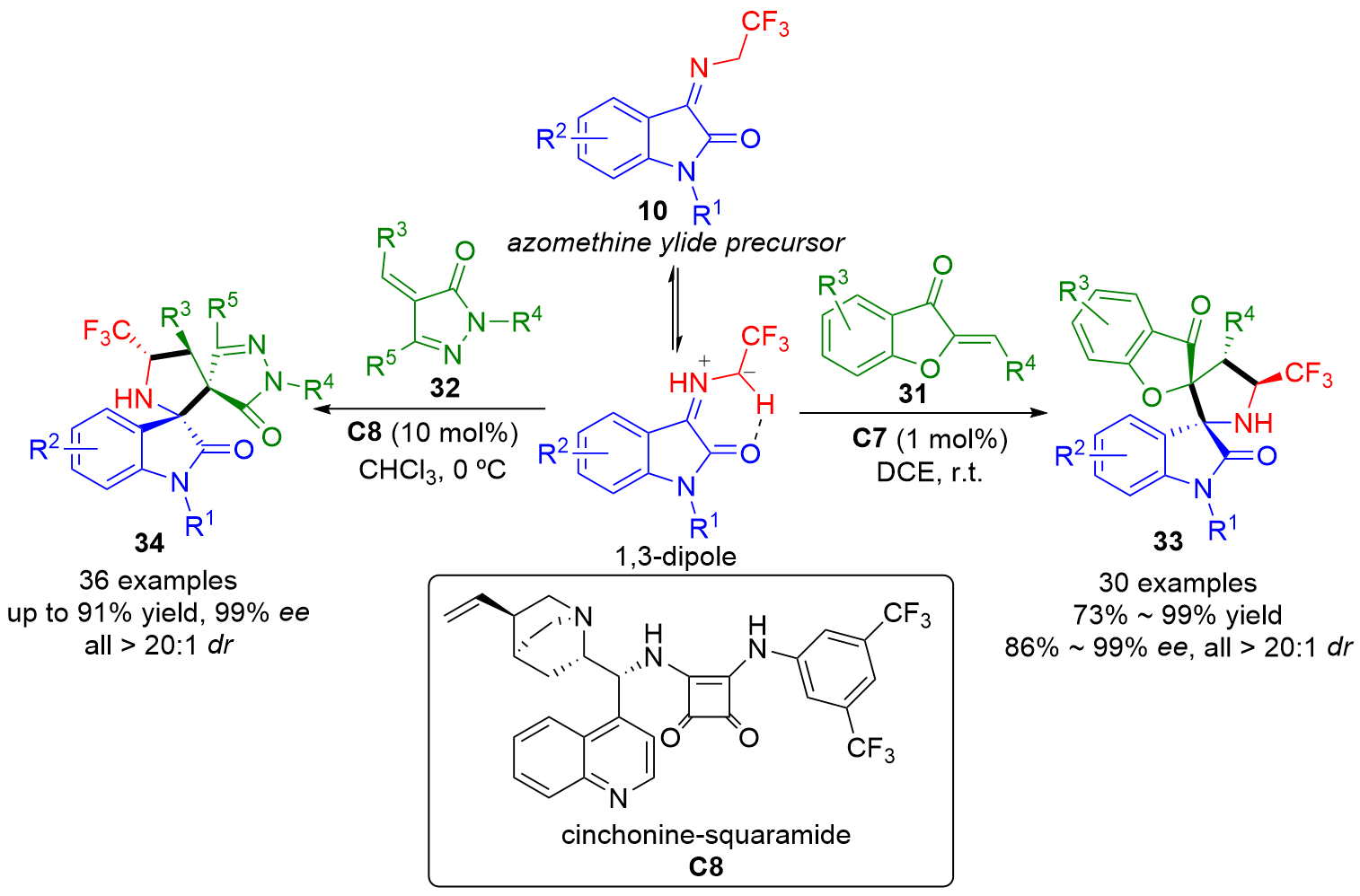





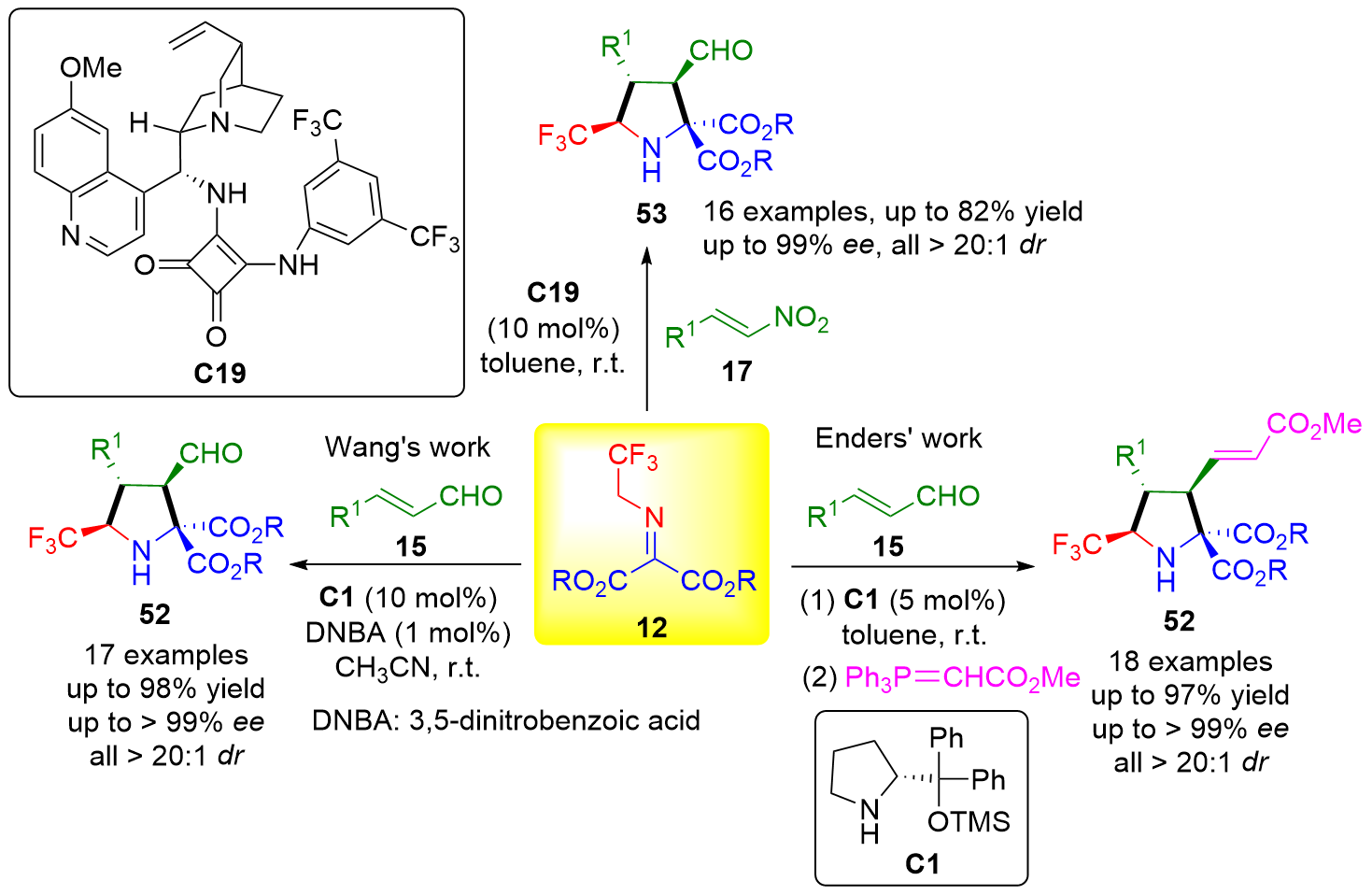
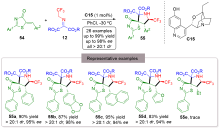

| [1] |
(a) Furuya, T.; Kamlet, A. S.; Ritter, T. Natrue 2011, 473, 470.
doi: 10.1038/nature10108 |
|
(b) Merino, F.; Nevado, C. Chem. Soc. Rev. 2014, 43, 6598.
doi: 10.1039/C4CS00025K |
|
|
(c) Usachev, B. I. J. Fluorine Chem. 2015, 175, 36.
doi: 10.1016/j.jfluchem.2015.02.009 |
|
|
(d) Huang, S.-C.; Schlinquer, C.; Poisson, T.; Pannecoucke, X.; Charette, A. B.; Jubault, P. Chem.-Eur. J. 2018, 24, 10339.
doi: 10.1002/chem.201802685 |
|
|
(d) He, X.-H.; Ji, Y.-L.; Peng, C.; Han, B. Adv. Synth. Catal. 2019, 361, 1923.
doi: 10.1002/adsc.v361.9 |
|
| [2] |
(a) Corbett, J. W.; Ko, S. S.; Rodgers, J. D.; Gearhart, L. A.; Magnus, N. A.; Bacheler, L. T.; Diamond, S.; Jeffrey, S.; Klabe, R. M.; Cordova, B. C.; Garber, S.; Logue, K.; Trainor, G. L.; Anderson, P. S.; Erickson-Viitanen, S. K. J. Med. Chem. 2000, 43, 2019.
pmid: 15115411 |
|
(b) Jlalia, I.; Lensen, N.; Chaume, G.; Dzhambazova, E.; Astasidi, L.; Hadjiolova, R.; Bocheva, A.; Brigaud, T. Eur. J. Med. Chem. 2013, 62, 122.
doi: 10.1016/j.ejmech.2012.12.041 pmid: 15115411 |
|
|
(c) Liu, Y.; Chen, J.-L.; Wang, G.-H.; Sun, P.; Huang, H.; Qing, F.-L. Tetrahedron Lett. 2013, 54, 5541.
doi: 10.1016/j.tetlet.2013.08.027 pmid: 15115411 |
|
|
(d) Guillaume, M.; Benoit, C.; Sebastien, C.; Philippe, G.; Pierre, B. J.; Daniele, B. D. J. Med. Chem. 2004, 47, 2694.
pmid: 15115411 |
|
|
(e) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455.
doi: 10.1021/cr100166a pmid: 15115411 |
|
| [3] |
(a) Timperley, C.M. Waters, M. J. Fluorine Chem. 2005, 126, 1144.
doi: 10.1016/j.jfluchem.2005.04.016 |
|
(b) Morandi, B.; Carreira, C. M. Angew. Chem., Int. Ed. 2010, 49, 119.
|
|
|
(c) Morandi, B.; Mariampillai, B.; Carreira, C. M. Angew. Chem., Int. Ed. 2011, 50, 1101.
|
|
|
(d) Li, F.; Nie, J.; Dun, L.; Zheng, Y.; Ma, J.-A. Angew. Chem., Int. Ed. 2013, 52, 6255.
doi: 10.1002/anie.201301870 |
|
|
(e) Molander, G. A.; Ryu, D. Angew. Chem., Int. Ed. 2014, 53, 14181.
doi: 10.1002/anie.v53.51 |
|
|
(f) Brusoe, A.T; Hartwig, J. F. J. Am. Chem. Soc. 2015, 137, 8460.
doi: 10.1021/jacs.5b02512 |
|
|
(g) Li, S.; Cao, W.-J.; Ma, J.-A. Synlett 2017, 28, 673.
doi: 10.1055/s-0036-1588363 |
|
|
(h) Kotozaki, M.; Chanthamath, S.; Fujii, T.; Shibatomi, K.; Iwasa, S. Chem. Commun. 2018, 54, 5110.
doi: 10.1039/C8CC02286K |
|
|
(i) Zhang, X.-W.; Hu, W.-L.; Chen, S.; Hu, X.-G. Org. Lett. 2018, 20, 860.
doi: 10.1021/acs.orglett.7b04028 |
|
|
(j) Gui, H.-Z.; Wei, Y.; Shi, M. Chem.-Asian J. 2020, 15, 1225.
doi: 10.1002/asia.v15.8 |
|
| [4] |
Ma, M.-X.; Zhu, Y.-Y.; Sun, Q.-T.; Li, X.-Y.; Su, J.-H.; Zhao, L.; Zhap, Y.-Y.; Qiu, S.; Yan, W.-J.; Wang, K.-R.; Wang, R. Chem. Commun. 2015, 51, 8789.
doi: 10.1039/C4CC10216A |
| [5] |
Zhi, Y.; Zhao, K.; Liu, Q.; Wang, A.; Enders, D. Chem. Commun. 2016, 52, 14011.
doi: 10.1039/C6CC08352H |
| [6] |
Dong, Z.-H.; Zhu, Y.-Y.; Li, B.-Y.; Wang, C.; Yan, W.-J.; Wang, K.-R.; Wang, R. J. Org. Chem. 2017, 82, 3482.
doi: 10.1021/acs.joc.6b02949 |
| [7] |
Sun, Q.-T.; Li, X.-Y.; Su, J.-H.; Zhao, L.; Ma, M.-X.; Zhu, Y.-Y.; Zhao, Y.-Y.; Zhu, R.-R.; Yan, W.-J.; Wang, K.-R.; Wang, R. Adv. Synth. Catal. 2015, 357, 3187.
doi: 10.1002/adsc.201500416 |
| [8] |
You, Y.; Lu, W.-Y.; Wang, Z.-H.; Chen, Y.-Z.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2018, 20, 4453.
doi: 10.1021/acs.orglett.8b01730 |
| [9] |
Gao, X.-Y.; Yan, R.-J.; Xiao, B.-X.; Du, W.; Albrecht, L.; Chen, Y.-C. Org. Lett. 2019, 21, 9628.
doi: 10.1021/acs.orglett.9b03794 |
| [10] |
Zhou, C.-C.; Han, Y.-Y.; Zeng, C.-K.; Zhang, T.-Y.; Ye, J.-X. Chin. Chem. Lett. 2020, 31, 377.
doi: 10.1016/j.cclet.2019.07.052 |
| [11] |
Choudhury, A.R; Mukherjee, S. Chem. Soc. Rev. 2020, 49, 6755.
doi: 10.1039/c9cs00346k pmid: 32785345 |
| [12] |
Wang, Z.-H.; Wu, Z.-J.; Yue, D.-F.; Hu, W.-F.; Zhang, X.-M.; Xu, X.-Y.; Yuan, W.-C. Chem. Commun. 2016, 52, 11708.
doi: 10.1039/C6CC06367E |
| [13] |
(a) Song, Y.-X.; Du, D.-M. J. Org. Chem. 2018, 83, 9278.
doi: 10.1021/acs.joc.8b01245 |
|
(b) Lin, Y.; Song, Y.-X.; Du, D.-M. Adv. Synth. Catal. 2019, 361, 1064.
doi: 10.1002/adsc.201801608 |
|
|
(c) An, T.-L.; Du, D.-M. ChemistrySelect 2019, 4, 11302.
doi: 10.1002/slct.v4.38 |
|
| [14] |
(a) Li, B.-Y.; Gao, F.-Y.; Feng, X.; Sun, M.-M.; Guo, Y.-F.; Wen, D.-W.; Deng, Y.-B.; Huang, J.-Q.; Wang, K.-R.; Yan, W.-J. Org. Chem. Front. 2019, 6, 1567.
doi: 10.1039/C9QO00241C |
|
(b) Wang, C.; Wen, D.-W.; Chen, H.; Deng, Y.-B.; Liu, X.-T.; Liu, X.; Wang, L.; Gao, F.-Y.; Guo, Y.-F.; Sun, M.-M.; Wang, K.-R.; Yan, W.-J. Org. Biomol. Chem. 2019, 17, 5514.
doi: 10.1039/c9ob00720b |
|
| [15] |
Zhao, X.-Y.; Xiong, J.-L.; An, J.-K.; Yu, J.-C.; Zhu, L.-P.; Feng, X.; Jiang, X.-X. Org. Chem. Front. 2019, 6, 1989.
doi: 10.1039/C9QO00452A |
| [16] |
Liu, X.; Lu, D.-M.; Wu, J.-H.; Tan, J.-P.; Jiang, C.-H.; Gao, G.-W.; Wang, T.-L. Adv. Synth. Catal. 2020, 362, 1490.
doi: 10.1002/adsc.v362.7 |
| [17] |
(a) Sun, Q.-S.; Zhu, H.; Chen, Y.-J.; Yang, X.-D.; Sun, X.-W.; Lin, G.-Q. Angew. Chem., Int. Ed. 2015, 54, 13253.
doi: 10.1002/anie.v54.45 |
|
(b) Zhu, L.-Y.; Chen, Q.-L.; Shen, D.; Zhang, W.-H.; Shen, C.; Zeng, X.-F.; Zhong, G.-F. Org. Lett. 2016, 18, 2387.
doi: 10.1021/acs.orglett.6b00873 |
|
|
(c) Ren, J.-W.; Wang, J.; Xiao, J.-A.; Li, J.; Xiang, H.-Y.; Chen, X.-Q.; Yang, H. J. Org. Chem. 2017, 82, 6441.
doi: 10.1021/acs.joc.7b00733 |
|
|
(d) Yang, Q.-Q.; Xiao, W.; Du, W.; Qin, Q.-Y.; Chen, Y.-C. Chem. Commun. 2018, 54, 1129.
doi: 10.1039/C7CC09221K |
|
|
(e) Wang, C.-Y.; Wang, Z.-Y.; Yang, J.; Shi, S.-H.; Hui, X.-P. Org. Lett. 2020, 22, 4440.
doi: 10.1021/acs.orglett.0c01447 |
|
| [18] |
(a) Huang, W.-J.; Chen, Q.; Lin, N.; Long, X.-W.; Pan, W.-G.; Xiong, Y.-S.; Weng, J.; Lu, G. Org. Chem. Front. 2017, 4, 472.
doi: 10.1039/C6QO00723F |
|
(b) Zhi, Y.; Zhao, K.; Essen, C. V.; Rissanen, K.; Enders, D. Synlett 2017, 28, 2876.
doi: 10.1055/s-0036-1589070 |
|
|
(c) Zhao, B.-L.; Du, D.-M. Adv. Synth. Catal. 2019, 361, 3412.
doi: 10.1002/adsc.v361.14 |
|
|
(d) Li, Yang, Hua, Y.-Z.; Lu, H.-J.; Liu, L.-T.; Wan, M.-C. Org. Lett. 2020, 22, 2527.
doi: 10.1021/acs.orglett.0c00283 |
|
| [19] |
Zhu, W.-R.; Zhang, Z.-W.; Huang, W.-H.; Lin, N.; Chen, K.-B.; Wang, B.-C.; Weng, J.; Lu, G. Synthesis 2019, 51, 1969.
doi: 10.1055/s-0037-1612089 |
| [20] |
Li, X.-Y.; Sun, J.-H.; Liu, Z. -R.-J.; Zhu, Y.-Y.; Dong, Z.-H.; Qiu, S.; Wang, J.-Y.; Lin, L.; Shen, Z.-Q.; Yan, W.-J.; Wang, K.-R.; Wang, R. Org. Lett. 2016, 18, 956.
doi: 10.1021/acs.orglett.5b03566 |
| [21] |
Shi, L.-M.; Sun, X.-S.; Chen, C.; Wang, Z.-F.; Tao, H.-Y.; Wang, C.-J. Org. Lett. 2019, 21, 4842.
doi: 10.1021/acs.orglett.9b01738 |
| [22] |
Onywagusi, C. I.; Shao, X.-X.; Malcolmson, S. J. Org. Lett. 2020, 22, 681.
|
| [23] |
Zhu, W.-R.; Liu, K.; Weng, J.; Huang, W.-H.; Huang, W.-J.; Chen, Q.; Lin, N.; Lu, G. Org. Lett. 2020, 22, 5014.
doi: 10.1021/acs.orglett.0c01578 |
| [24] |
(a) Zhu, Y.; Buchwald, S. L. J. Am. Chem. Soc. 2014, 136, 4500.
doi: 10.1021/ja501560x |
|
(b) Liu, J.; Cao, C.-G.; Sun, H.-B.; Zhang, X.; Niu, D.-W. J. Am. Chem. Soc. 2016, 138, 13103.
doi: 10.1021/jacs.6b05288 |
|
| [25] |
(a) Wang, Y.-W.; Deng, L.-F.; Zhang, X.; Niu, D.-W. Org. Lett. 2019, 21, 6951.
doi: 10.1021/acs.orglett.9b02550 |
|
(b) Shen, C.; Wang, R.-Q.; Wei, L.; Wang, Z.-F.; Tao, H.-Y.; Wang, C.-J. Org. Lett. 2019, 21, 6940.
doi: 10.1021/acs.orglett.9b02543 |
|
| [26] |
Wang, W.; Xiong, Q.; Gong, L.; Wang, Y.-W.; Liu, J.; Lan, Y.; Zhang, X. Org. Lett. 2020, 22, 5479.
doi: 10.1021/acs.orglett.0c01836 |
| [27] |
Sun, J.-H.; Ma, Z.-L.; Li, X.-Y.; Li, L.; Shen, Z.-Q.; Yang, P.-J.; Li, Y.; Wang, H.-L.; Yan, W.-J.; Wang, K.-R.; Wang, R. Adv. Synth. Catal. 2016, 358, 3777.
doi: 10.1002/adsc.201600688 |
| [28] |
Liu, Q.; Zhao, K.; Zhi, Y.; Raabe, G.; Enders, D. Org. Chem. Front. 2017, 4, 1416.
doi: 10.1039/C7QO00161D |
| [29] |
Li, B.-Y.; Liu, J.-K.; Gao, F. Y.; Sun, M.-M.; Guo, Y.-F.; Zhou, Y.; Wen, D.-W.; Deng, Y.-B.; Chen, H.; Wang, K.-R.; Yan, W.-J. Org. Biomol. Chem. 2019, 17, 2892.
doi: 10.1039/C9OB00325H |
| [30] |
Zhu, W.-Y.; Su, Q.; Lin, N.; Chen, Q.; Zhang, Z.-W.; Weng, J.; Lu, G. Org. Chem. Front. 2020, 7, 3452.
doi: 10.1039/D0QO00990C |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [4] | 文思, 丁宇浩, 田青于, 葛进, 程国林. 铑(III)催化苯甲亚胺酸乙酯和CF3-亚胺氧锍叶立德C—H 活化/环化反应合成CF3-1H-苯并[de][1,8]萘吡啶[J]. 有机化学, 2024, 44(1): 291-300. |
| [5] | 陈祖良, 魏颖静, 张俊良. 供体-受体氮杂环丙烷碳-碳键断裂的环加成反应研究进展[J]. 有机化学, 2023, 43(9): 3078-3088. |
| [6] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [7] | 马虎, 黄丹凤, 王克虎, 唐朵朵, 冯杨, 任园园, 王君娇, 胡雨来. 3-(三氟甲基)吡唑类化合物的合成[J]. 有机化学, 2023, 43(9): 3257-3267. |
| [8] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [9] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [10] | 孔德亮, 戴闻, 赵怡玲, 陈艺林, 朱红平. 脒基胺硼基硅宾与单酮和二酮的氧化环加成反应研究[J]. 有机化学, 2023, 43(5): 1843-1851. |
| [11] | 刘静, 郝健, 沈其龙. 可见光促进的含色氨酸寡肽与YlideFluor试剂的直接三氟甲基化反应研究[J]. 有机化学, 2023, 43(4): 1517-1524. |
| [12] | 戴春波, 夏思奇, 陈晓玉, 杨丽敏. 氮杂环卡宾(NHC)催化[4+3]环加成反应构建4-氨基苯并环庚烯内酯[J]. 有机化学, 2023, 43(3): 1084-1090. |
| [13] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [14] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [15] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||