有机化学 ›› 2021, Vol. 41 ›› Issue (11): 4467-4475.DOI: 10.6023/cjoc202105002 上一篇 下一篇
研究论文
收稿日期:2021-05-01
修回日期:2021-07-05
发布日期:2021-07-26
通讯作者:
黄杰, 付振乾
基金资助:
Yuxia Zhanga, Jingcheng Guoa, Jie Huangb( ), Zhenqian Fua(
), Zhenqian Fua( )
)
Received:2021-05-01
Revised:2021-07-05
Published:2021-07-26
Contact:
Jie Huang, Zhenqian Fu
Supported by:文章分享
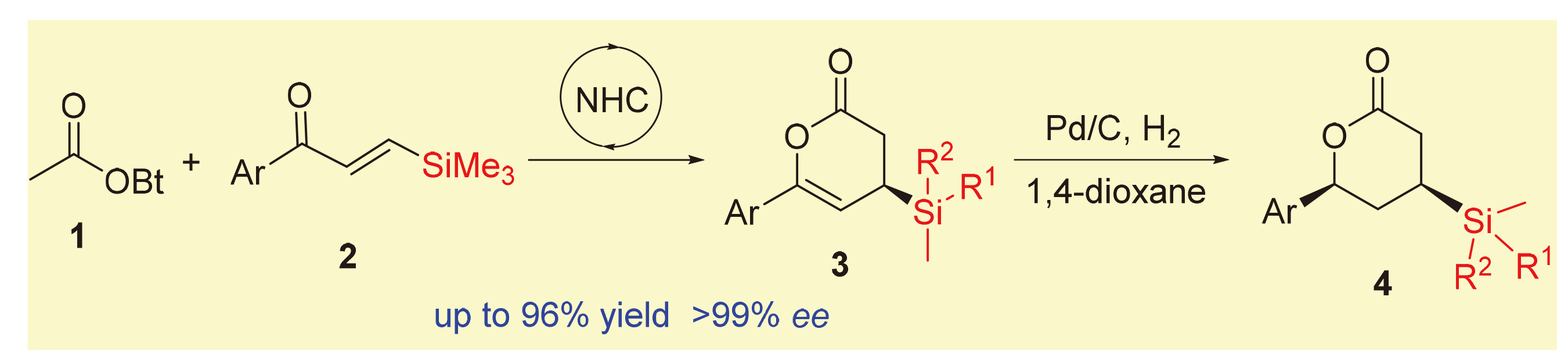
在氮杂环卡宾催化下, 乙酸酯和β-硅基烯酮发生[4+2]环合反应, 可高立体选择性地合成具有潜在应用价值的β-硅基δ-内酯类化合物. 该方法具有底物简单易得、反应条件温和、底物普适性好和操作简单等优点, 且反应规模放大10倍后也得到了优异的产率和对映体选择性. 该反应产物在氢化还原反应中均呈现出优良的实验结果, 可转化为降血脂药Ezetimibe.
张雨霞, 郭京程, 黄杰, 付振乾. 氮杂环卡宾催化乙酸酯和β-硅基烯酮的[4+2]环合反应:高立体选择性合成β-硅基δ-内酯[J]. 有机化学, 2021, 41(11): 4467-4475.
Yuxia Zhang, Jingcheng Guo, Jie Huang, Zhenqian Fu. N-Heterocyclic Carbene-Catalyzed [4+2] Annulation of Acetates and β-Silyl Enones: Highly Enantioselective Synthesis of β-Silyl δ-Lactones[J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4467-4475.
| Entry | NHC | 1 | Base | Solvent | Yieldb/% | eec/% |
|---|---|---|---|---|---|---|
| 1 | A | 1a | DBU | THF | 44 | — |
| 2 | B | 1a | DBU | THF | 17 | 99 |
| 3 | B | 1b | DBU | THF | 88 | 99 |
| 3 | B | 1b | DBU | 1,4-Dioxane | 55 | 99 |
| 4 | B | 1b | DBU | CH3CN | 54 | 99 |
| 5 | B | 1b | DBU | TOL | 66 | 99 |
| 6 | B | 1b | DBU | DCM | 66 | 99 |
| 7 | B | 1b | DBU | CHCl3 | 57 | 99 |
| 8 | B | 1b | Et3N | THF | 50 | 99 |
| 9 | B | 1b | DIPEA | THF | 49 | 99 |
| 10 | B | 1b | K2CO3 | THF | 40 | 99 |
| 11 | B | 1b | DMAP | THF | 51 | 99 |
| 12 | B | 1b | DBN | THF | 85 | 99 |
| 13d | B | 1b | DBU | THF | 86 | 99 |
| 14e | B | 1b | DBU | THF | 74 | 99 |
| 15f | B | 1b | DBU | THF | 69 | 99 |
| 16d,g | B | 1b | DBU | THF | 80 | 99 |
| Entry | NHC | 1 | Base | Solvent | Yieldb/% | eec/% |
|---|---|---|---|---|---|---|
| 1 | A | 1a | DBU | THF | 44 | — |
| 2 | B | 1a | DBU | THF | 17 | 99 |
| 3 | B | 1b | DBU | THF | 88 | 99 |
| 3 | B | 1b | DBU | 1,4-Dioxane | 55 | 99 |
| 4 | B | 1b | DBU | CH3CN | 54 | 99 |
| 5 | B | 1b | DBU | TOL | 66 | 99 |
| 6 | B | 1b | DBU | DCM | 66 | 99 |
| 7 | B | 1b | DBU | CHCl3 | 57 | 99 |
| 8 | B | 1b | Et3N | THF | 50 | 99 |
| 9 | B | 1b | DIPEA | THF | 49 | 99 |
| 10 | B | 1b | K2CO3 | THF | 40 | 99 |
| 11 | B | 1b | DMAP | THF | 51 | 99 |
| 12 | B | 1b | DBN | THF | 85 | 99 |
| 13d | B | 1b | DBU | THF | 86 | 99 |
| 14e | B | 1b | DBU | THF | 74 | 99 |
| 15f | B | 1b | DBU | THF | 69 | 99 |
| 16d,g | B | 1b | DBU | THF | 80 | 99 |
| [1] |
(a) Chan, T. H.; Wang, D. Chem. Rev. 1992, 92, 995.
doi: 10.1021/cr00013a012 pmid: 26478695 |
|
(b) Masse, C. E.; Panek, J. S. Chem. Rev. 1995, 95, 1293.
doi: 10.1021/cr00037a008 pmid: 26478695 |
|
|
(c) Fleming, I.; Barbero, A.; Walter, D. Chem. Rev. 1997, 97, 2063.
pmid: 26478695 |
|
|
(d) Denmark, S. E.; Regens, C. S. Acc. Chem. Res. 2008, 41, 1486.
doi: 10.1021/ar800037p pmid: 26478695 |
|
|
(e) Nakao, Y.; Hiyama, T. Chem. Soc. Rev. 2011, 40, 4893.
doi: 10.1039/c1cs15122c pmid: 26478695 |
|
|
(f) Zhang, H. J.; Priebbenow, D. L.; Bolm, C. Chem. Soc. Rev. 2013, 42, 8540.
doi: 10.1039/c3cs60185d pmid: 26478695 |
|
|
(g) Denmark, S. E.; Ambrosi, A. Org. Process Res. Dev. 2015, 19, 982.
pmid: 26478695 |
|
|
(h) Komiyama, T.; Minami, Y.; Hiyama, T. ACS Catal. 2017, 7, 631.
doi: 10.1021/acscatal.6b02374 pmid: 26478695 |
|
|
(i) Cui, Y. M.; Lin, Y.; Xu, L. W. Coord. Chem. Rev. 2017, 330, 37.
doi: 10.1016/j.ccr.2016.09.011 pmid: 26478695 |
|
| [2] |
(a) Patai, S.; Rappoport, Z.; Ojima, I. The Chemistry of Organic Silicon Compounds, Chichester, Wiley, 1989.
|
|
(b) Brook, M. A. Silicon in Organic, Organometallic, and Polymer Chemistry, New York, Wiley, 1999.
|
|
| [3] |
Cash, G. G. Pestic. Sci. 1997, 49, 29.
doi: 10.1002/(SICI)1096-9063(199701)49:1【-逻*辑*与-】#x00026;lt;【-逻*辑*与-】#x00026;gt;1.0.CO;2-I |
| [4] |
(a) Mutahi, M. W.; Nittoli, T.; Guo, L.; Sieburth, S. M. J. Am. Chem. Soc. 2002, 124, 7363.
doi: 10.1021/ja026158w |
|
(b) Bains, W.; Tacke, R. Curr. Opin. Drug Discovery Dev. 2003, 6, 526.
|
|
|
(c) Pooni, P. K.; Showell, G. A. Mini. Rev. Med. Chem. 2006, 6, 1169.
doi: 10.2174/138955706778560120 |
|
|
(d) Gately, S.; West, R. Drug Dev. Res. 2007, 68, 156.
doi: 10.1002/(ISSN)1098-2299 |
|
|
(e) Franz, A. K.; Wilson, S. O. J. Med. Chem. 2012, 56, 388.
doi: 10.1021/jm3010114 |
|
|
(f) Min, G. K.; Hernndez, D.; Skrydstrup, T. Acc. Chem. Res. 2013, 46, 457.
doi: 10.1021/ar300200h |
|
| [5] |
(a) Jensen, J. F.; Svendsen, B. Y.; la Cour, T. V.; Pedersen, H. L.; Johannsen, M. J. Am. Chem. Soc. 2002, 124, 4558.
pmid: 28654260 |
|
(b) Tang, X.; Xie, L.; Chen, Y.; Tian, P.; Lin, G. Chin. J. Org. Chem. 2016, 36, 2011. (in Chinese)
doi: 10.6023/cjoc201605004 pmid: 28654260 |
|
|
(唐小齐, 谢立波, 陈雅丽, 田平, 林国强, 有机化学, 2016, 36, 2011.)
doi: 10.6023/cjoc201605004 pmid: 28654260 |
|
|
(c) Cheng, B.; Lu, P.; Zhang, H.; Cheng, X.; Lu, Z. J. Am. Chem. Soc. 2017, 139, 9439.
doi: 10.1021/jacs.7b04137 pmid: 28654260 |
|
|
(d) Guo, J.; Shen, X.; Lu, Z. Angew. Chem. Int. Ed. 2017, 56, 615.
doi: 10.1002/anie.201610121 pmid: 28654260 |
|
|
(e) Dai, Z.; Yu, Z.; Bai, Y.; Li, J.; Peng, J. Chin. J. Org. Chem. 2020, 40, 1177. (in Chinese)
doi: 10.6023/cjoc201910012 pmid: 28654260 |
|
|
(代自男, 余泽浩, 白赢, 厉嘉云, 彭家建, 有机化学, 2020, 40, 1177.)
doi: 10.6023/cjoc201910012 pmid: 28654260 |
|
|
(f) Liu, Y.; Dong, X.; Zhang, X. Chin. J. Org. Chem. 2020, 40, 1096. (in Chinese)
doi: 10.6023/cjoc201912025 pmid: 28654260 |
|
|
(刘元华, 董秀琴, 张绪穆, 有机化学, 2020, 40, 1096.)
doi: 10.6023/cjoc201912025 pmid: 28654260 |
|
| [6] |
(a) Zhang, Y. Z.; Zhu, S. F.; Wang, L. X.; Zhou, Q. L. Angew. Chem. Int. Ed. 2008, 120, 8624.
doi: 10.1002/ange.v120:44 |
|
(b) Chen, D.; Zhu, D. X.; Xu, M. H. J. Am. Chem. Soc. 2016, 138, 1498.
doi: 10.1021/jacs.5b12960 |
|
|
(c) Zhang, H.; Li, L.; Shen, F.; Cai, T.; Shen, R. Chin. J. Org. Chem. 2020, 40, 873. (in Chinese)
doi: 10.6023/cjoc201911009 |
|
|
(张慧苗, 李灵芝, 沈方旗, 蔡涛, 沈润溥, 有机化学, 2020, 40, 873.)
doi: 10.6023/cjoc201911009 |
|
| [7] |
(a) Shintani, R.; Moriya, K.; Hayashi, T. J. Am. Chem. Soc. 2011, 133, 16440.
doi: 10.1021/ja208621x pmid: 22506681 |
|
(b) Shintani, R.; Otomo, H.; Ota, K.; Hayashi, T. J. Am. Chem. Soc. 2012, 134, 7305.
doi: 10.1021/ja302278s pmid: 22506681 |
|
|
(c) Zhang, Q.; An, K.; Liu, L. C.; Zhang, Q.; Guo, H.; He, W. Angew. Chem. Int. Ed. 2016, 55, 6319.
doi: 10.1002/anie.201602376 pmid: 22506681 |
|
|
(d) Su, B.; Harting, J. F. J. Am. Chem. Soc. 2017, 139, 12137.
doi: 10.1021/jacs.7b06679 pmid: 22506681 |
|
| [8] |
(a) Shintani, R.; Okamoto, K.; Hayashi, T. Org. Lett. 2005, 7, 4757.
pmid: 25323817 |
|
(b) Balskus, E. P.; Jacobsen, E. N. J. Am. Chem. Soc. 2006, 128, 6810.
pmid: 25323817 |
|
|
(c) Kacprzynski, M. A.; Kazane, S. A.; May, T. L.; Hoveyda, A. H. Org. Lett. 2007, 9, 3187.
pmid: 25323817 |
|
|
(d) Zhao, K.; Loh, T. P. Chem.-Eur. J. 2014, 20, 16764.
doi: 10.1002/chem.201403849 pmid: 25323817 |
|
| [9] |
(a) Chang, K. J.; Rayabarpu, D. K.; Yang, F. Y.; Cheng, C. H. J. Am. Chem. Soc. 2005, 127, 126.
doi: 10.1021/ja044662q pmid: 26646601 |
|
(b) Ohmura, T.; Tamlguchi, H.; Suginome, M. J. Am. Chem. Soc. 2006, 128, 13682.
doi: 10.1021/ja063934h pmid: 26646601 |
|
|
(c) Lee, K. S.; Hoveyda, A. H. J. Am. Chem. Soc. 2010, 132, 2898.
doi: 10.1021/ja910989n pmid: 26646601 |
|
|
(d) Gilles, P.; Py, S. Org. Lett. 2012, 14, 1042.
doi: 10.1021/ol203396s pmid: 26646601 |
|
|
(e) Pace, V.; Rae, J. P.; Harb, H. Y.; Procter, D. J. Chem. Commun. 2013, 49, 5150.
doi: 10.1039/c3cc42160k pmid: 26646601 |
|
|
(f) Kitanosono, T.; Zhu, L.; Liu, C.; Xu, P.; Kobayashi, S. J. Am. Chem. Soc. 2015, 137, 15422.
doi: 10.1021/jacs.5b11418 pmid: 26646601 |
|
| [10] |
O'brien, J. M.; Hoveyda, A. H. J. Am. Chem. Soc. 2011, 133, 7712.
doi: 10.1021/ja203031a |
| [11] |
(a) Zhang, Y. X.; Huang, J.; Guo, Y. Y.; Li, L.; Fu, Z. Q.; Huang, W. Angew. Chem. Int. Ed. 2018, 57, 4594.
doi: 10.1002/anie.v57.17 |
|
(b) Zhang, Y. X.; Huang, X.; Guo, J. C.; Wei, C. L.; Gong, M. H.; Fu, Z. Q. Org. Lett. 2020, 22, 9545.
doi: 10.1021/acs.orglett.0c03589 |
|
| [12] |
(a) Florence, G. J.; Gardner, N. M.; Paterson, I. Nat. Prod. Rep. 2008, 25, 342.
doi: 10.1039/b705661n pmid: 18389141 |
|
(b) Mondon, M.; Gesson, J. P. Curr. Org. Synth. 2006, 3, 41.
doi: 10.2174/157017906775473966 pmid: 18389141 |
|
|
(c) Chiu, P.; Leung, L. T.; Ko, C. B. B. Nat. Prod. Rep. 2010, 27, 1066.
doi: 10.1039/b906520m pmid: 18389141 |
|
| [13] |
Selected reviews on NHC catalysis: (a) Enders, D.;,. Enders, D.; Balensiefer, T. Acc. Chem. Res. 2004, 37, 534.
doi: 10.1021/ar030050j pmid: 25992594 |
|
(b) Enders, D.; Niemeier, O.; Henseler, A. Chem. Rev. 2007, 107, 5606.
doi: 10.1021/cr068372z pmid: 25992594 |
|
|
(c) Nair, V.; Menon, R. S.; Biju, A. T.; Sinu, C. R.; Paul, R. R.; Jose, A.; Sreekumar, V. Chem. Soc. Rev. 2011, 40, 5336.
doi: 10.1039/c1cs15139h pmid: 25992594 |
|
|
(d) Izquierdo, J.; Hutson, G. E.; Cohen, D. T.; Scheidt, K. A. Angew. Chem. Int. Ed. 2012, 51, 11686.
doi: 10.1002/anie.201203704 pmid: 25992594 |
|
|
(e) Douglas, J.; Churchill, G.; Smith, A. D. Synthesis 2012, 44, 2295.
doi: 10.1055/s-00000084 pmid: 25992594 |
|
|
(f) Ryan, S. J.; Candish, L.; Lupton, D. W. Chem. Soc. Rev. 2013, 42, 4906.
doi: 10.1039/c3cs35522e pmid: 25992594 |
|
|
(g) De Sarkar, S.; Biswas, A.; Samanta, R. C.; Studer, A. Chem.- Eur. J. 2013, 19, 4664.
pmid: 25992594 |
|
|
(h) FHvre, M.; Pinaud, J.; Gnanou, Y.; Vignolle, J.; Taton, D. Chem. Soc. Rev. 2013, 42, 2142.
doi: 10.1039/c2cs35383k pmid: 25992594 |
|
|
(i) Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485.
doi: 10.1038/nature13384 pmid: 25992594 |
|
|
(j) Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115, 9307.
doi: 10.1021/acs.chemrev.5b00060 pmid: 25992594 |
|
|
(k) Menon, R. S.; Biju, A. T.; Nair, V. Chem. Soc. Rev. 2015, 44, 5040.
doi: 10.1039/C5CS00162E pmid: 25992594 |
|
|
(l) Menon, R. S.; Biju, A. T.; Nair, V. Beilstein J. Org. Chem. 2016, 12, 444.
doi: 10.3762/bjoc.12.47 pmid: 25992594 |
|
|
(m) Zhang, C.; Hooper, J. F.; Lupton, D. W. ACS Catal. 2017, 7, 2583.
doi: 10.1021/acscatal.6b03663 pmid: 25992594 |
|
|
(n) Chen, X. Y.; Gao, Z. H.; Ye, S. Acc. Chem. Res. 2020, 53, 690.
doi: 10.1021/acs.accounts.9b00635 pmid: 25992594 |
|
|
(o) Ohmiya, H. ACS Catal. 2020, 10, 6862.
doi: 10.1021/acscatal.0c01795 pmid: 25992594 |
|
|
(p) Chen, X.; Wang, H.; Jin, Z.; Chi, Y. R. Chin. J. Chem. 2020, 38, 1167.
doi: 10.1002/cjoc.v38.10 pmid: 25992594 |
|
| [14] |
Hua, Y. Y.; Bin, H. Y.; Wei, T.; Cheng, H. A.; Lin, Z. P.; Fu, X. F.; Li, Y. Q.; Xie, J. H.; Yan, P. C.; Zhou, Q. L. Org. Lett. 2020, 22, 818.
doi: 10.1021/acs.orglett.9b04253 |
| [15] |
(a) Brink, B. D.; DeFrancisco, J. R.; Hillner, J A.; Linton, B. R. J. Org. Chem. 2011, 76, 5258.
doi: 10.1021/jo200346r |
|
(b) Pradhan, N.; Paul, S.; Deka, S. J.; Roy, A.; trivedi, V.; Manna, D. ChemSelect 2017, 2, 5511.
|
| [1] | 夏登鹏, 罗锦昀, 何林, 蔡志华, 杜广芬. 氮杂环卡宾催化的五氟苯基硫醚的合成[J]. 有机化学, 2024, 44(2): 622-630. |
| [2] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [3] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [4] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [5] | 蔡远林, 吕亚, 聂桂花, 金智超, 池永贵. 氮杂环卡宾催化合成氰基化合物的研究进展[J]. 有机化学, 2023, 43(9): 3135-3145. |
| [6] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [7] | 秦娇, 陈杰, 苏艳. 无过渡金属催化的α-溴代茚酮自由基裂解反应合成(2-氰基苯基)乙酸-2,2,6,6-四甲基哌啶酯[J]. 有机化学, 2023, 43(6): 2171-2177. |
| [8] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [9] | 杨亮茹, 郭梦丽, 袁金伟, 王佳美, 夏宇婷, 肖咏梅, 毛璞. 钳形氮杂环卡宾金属络合物的研究进展[J]. 有机化学, 2023, 43(6): 2002-2025. |
| [10] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [11] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [12] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [13] | 戴春波, 夏思奇, 陈晓玉, 杨丽敏. 氮杂环卡宾(NHC)催化[4+3]环加成反应构建4-氨基苯并环庚烯内酯[J]. 有机化学, 2023, 43(3): 1084-1090. |
| [14] | 刘婷婷, 胡宇才, 沈安. 亚胺配体协同氮杂环卡宾钯配合物催化碳碳偶联反应的作用机制[J]. 有机化学, 2023, 43(2): 622-628. |
| [15] | 赵佳怡, 葛怡聪, 何川. 不对称催化Si—H/X—H脱氢偶联构筑硅中心手性[J]. 有机化学, 2023, 43(10): 3352-3366. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||