有机化学 ›› 2021, Vol. 41 ›› Issue (3): 1207-1215.DOI: 10.6023/cjoc202009020 上一篇 下一篇
所属专题: 热点论文虚拟合集
研究论文
收稿日期:2020-09-08
修回日期:2020-10-02
发布日期:2020-10-28
通讯作者:
刘晓涛, 叶龙武
基金资助:
Xiaotao Liua,*( ), Xin Liub, Longwu Yeb,c,*(
), Xin Liub, Longwu Yeb,c,*( )
)
Received:2020-09-08
Revised:2020-10-02
Published:2020-10-28
Contact:
Xiaotao Liu, Longwu Ye
About author:Supported by:文章分享
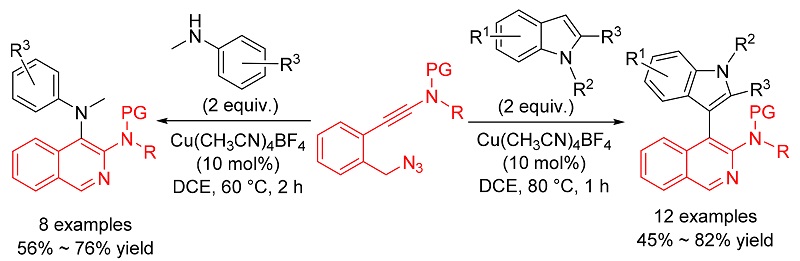
报道了利用铜催化叠氮-炔酰胺的环化反应合成异喹啉衍生物的方法. 首先通过铜催化分子内叠氮-炔酰胺环化反应生成α-亚胺铜卡宾中间体, 随后再分别被分子间的吲哚和苯胺捕获生成相应的C—H和N—H插入产物. 该方法操作简便、反应条件温和、底物普适性广, 为含有异喹啉-吲哚和异喹啉-苯胺骨架的天然产物和活性分子的合成提供了一条简便和高效的途径.
刘晓涛, 刘鑫, 叶龙武. 基于叠氮-炔酰胺环化的铜催化碳氢键和氮氢键插入反应研究[J]. 有机化学, 2021, 41(3): 1207-1215.
Xiaotao Liu, Xin Liu, Longwu Ye. Copper-Catalyzed C—H Bond and N—H Bond Insertion Reaction Based on Azide-Ynamide Cyclization[J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1207-1215.
| Entry | Catalyst | Condition | Yieldb/% 3a | Yieldb/% 3a'° |
|---|---|---|---|---|
| 1 | Cu(CH3CN)4PF6 | DCE, 60 ℃, 2 h | 52 | 35 |
| 2 | Cu(CH3CN)4BF4 | DCE, 60 ℃, 2 h | 58 | 28 |
| 3 | Cu(OTf) | DCE, 60 ℃, 2.5 h | 42 | 50 |
| 4 | CuCl | DCE, 60 ℃, 8 h | 40 | 46 |
| 5 | Cu(CH3CN)4BF4 | CHCl3, 60 ℃, 2 h | 57 | 33 |
| 6 | Cu(CH3CN)4BF4 | Toluene, 60 ℃, 6 h | 60 | 29 |
| 7 | Cu(CH3CN)4BF4 | DCE, 40 ℃, 16 h | 64 | 32 |
| 8 | Cu(CH3 CN)4 BF4 | DCE, 80 ℃, 1 h | 83 | <10 |
| 9c | Cu(CH3CN)4BF4 | DCE, 80 ℃, 2 h | 64 | 18 |
| 10d | Cu(CH3CN)4BF4 | DCE, 80 ℃, 1 h | 84 | <10 |
| Entry | Catalyst | Condition | Yieldb/% 3a | Yieldb/% 3a'° |
|---|---|---|---|---|
| 1 | Cu(CH3CN)4PF6 | DCE, 60 ℃, 2 h | 52 | 35 |
| 2 | Cu(CH3CN)4BF4 | DCE, 60 ℃, 2 h | 58 | 28 |
| 3 | Cu(OTf) | DCE, 60 ℃, 2.5 h | 42 | 50 |
| 4 | CuCl | DCE, 60 ℃, 8 h | 40 | 46 |
| 5 | Cu(CH3CN)4BF4 | CHCl3, 60 ℃, 2 h | 57 | 33 |
| 6 | Cu(CH3CN)4BF4 | Toluene, 60 ℃, 6 h | 60 | 29 |
| 7 | Cu(CH3CN)4BF4 | DCE, 40 ℃, 16 h | 64 | 32 |
| 8 | Cu(CH3 CN)4 BF4 | DCE, 80 ℃, 1 h | 83 | <10 |
| 9c | Cu(CH3CN)4BF4 | DCE, 80 ℃, 2 h | 64 | 18 |
| 10d | Cu(CH3CN)4BF4 | DCE, 80 ℃, 1 h | 84 | <10 |
| [1] |
For selected reviews, see: (a) Khan, A. Y.; Suresh Kumar, G. Biophys. Rev. 2015, 7, 407.
doi: 10.1007/s12551-015-0183-5 pmid: 15162226 |
|
(b) Iranshahy, M.; Quinn, R. J.; Iranshahi, M. RSC Adv. 2014, 4, 15900.
pmid: 15162226 |
|
|
(c) Bentley, K. W. Nat. Prod. Rep. 2006, 23, 444.
doi: 10.1039/b509523a pmid: 15162226 |
|
|
(d) Bentley, K. W. Nat. Prod. Rep. 2005, 22, 249.
pmid: 15162226 |
|
|
(e) Bentley, K. W. Nat. Prod. Rep. 2004, 21, 395.
pmid: 15162226 |
|
|
(f) Bentley, K. W. The Isoquinoline Alkaloids, Hardwood Academic, Amsterdam, 1998, Vol. 1.
pmid: 15162226 |
|
|
(g) Phillipson, J. D.; Roberts, M. F.; Zenk, M. H. The Chemistry and Biology of Isoquinoline Alkaloids, Springer Verlag, Berlin, 1985.
pmid: 15162226 |
|
| [2] |
(a) Wang, S.-B.; Wang, X.-F.; Qin, B.; Ohkoshi, E.; Hsieh, K.-Y.; Hamel, E.; Cui, M.-T.; Zhu, D.-Q.; Goto, M.; Morris-Natschke, S. L.; Lee, K.-H.; Xie, L. Bioorg. Med. Chem. 2015, 23, 5740.
pmid: 26242242 |
|
(b) Smith, N. D.; Bonnefous, C.; Zhuang, H.; Chen, X.; Duron, S.; Lindstrom, A. WO 2009029617, 2009.
pmid: 26242242 |
|
|
(c) Arrington, K. L.; Dudkin, V. Y.; Fraley, M. E.; Garbaccio, R. M.; Hartman, G. D.; Huang, S. Y.; Kreatsoulas, C.; Tasber, E. S. WO 2007008502, 2007.
pmid: 26242242 |
|
|
(d) Allen, J. R.; Amegadzie, A. K.; Gardinier, K. M.; Gregory, G. S.; Hitchcock, S. A.; Hoogestraat, P. J.; Jones, W. D. Jr; Smith, D. L. WO 2005066126, 2005.
pmid: 26242242 |
|
|
(e) Hopper, A.; Schumacher, R. A.; Tehim, A.; De Vivo, M.; Brubaker, W. F. Jr.; Liu, R.; Hess, H.-J. E.; Unterbeck, A. WO 2002074726, 2002. .
pmid: 26242242 |
|
| [3] |
For recent selected reviews on the generation of metal carbenes from alkynes, see: (a) Ye, L.-W.; Zhu, X.-Q.; Sahani, R. L.; Xu, Y.; Qian, P.-C.; Liu, R.-S. Chem. Rev. 2021, 121, DOI: 10.1021/acs.chemrev.0c00348.
pmid: 24428596 |
|
(b) Sahani, R. L.; Ye, L.-W.; Liu, R.-S. Adv. Organomet. Chem. 2020, 73, 195.
pmid: 24428596 |
|
|
(c) Chen, L.; C hen, K; Zhu, S. Chem 2018, 4, 1208.
pmid: 24428596 |
|
|
Liao, Y.; Zhu, L.; Yu, Y.; Chen, G.; Huang, X. Chin. J. Org. Chem. 2017, 37, 2785. (in Chinese)
doi: 10.6023/cjoc201708021 pmid: 24428596 |
|
|
(廖云, 朱磊, 俞颖华, 陈贵, 黄学良, 有机化学, 2017, 37, 2785.)
pmid: 24428596 |
|
|
(e) Zi, W.; Toste, F. D. Chem. Soc. Rev. 2016, 45, 4567.
pmid: 24428596 |
|
|
(f) Harris, R. J.; Widenhoefer, R. A. Chem. Soc. Rev. 2016, 45, 4533.
pmid: 24428596 |
|
|
(g) Zheng, Z.; Wang, Z.; Wang, Y.; Zhang, L. Chem. Soc. Rev. 2016, 45, 4448.
doi: 10.1039/c5cs00887e pmid: 24428596 |
|
|
(h) Jia, M.; Ma, S. Angew. Chem., Int. Ed. 2016, 55, 9134.
pmid: 24428596 |
|
|
(i) Dorel, R.; Echavarren, A. M. Chem. Rev. 2015, 115, 9028.
doi: 10.1021/cr500691k pmid: 24428596 |
|
|
(j) Wang, Y.; Muratore, M. E.; Echavarren, A. M. Chem.-Eur. J. 2015, 21, 7332.
doi: 10.1002/chem.201406318 pmid: 24428596 |
|
|
(k) Qian, D.; Zhang, J. Chem. Soc. Rev. 2015, 44, 667.
pmid: 24428596 |
|
|
(l) Zhang, L. Acc. Chem. Res. 2014, 47, 877.
doi: 10.1021/ar400181x pmid: 24428596 |
|
| [4] |
For reviews, see: (a) Aguilar, E.; Santamaría, J. Org. Chem. Front. 2019, 6, 1513.
doi: 10.1039/C9QO00243J |
|
(b) Davies, P. W.; Garzón, M. Asian J. Org. Chem. 2015, 4, 694.
doi: 10.1002/ajoc.v4.8 |
|
| [5] |
For selected examples, see: (a) Zhu, X.-Q.; Wang, Z.-S.; Hou, B.-S.; Zhang, H.-W.; Deng, C.; Ye, L.-W. Angew. Chem., Int. Ed. 2020, 59, 1666.
pmid: 29560212 |
|
(b) Tian, X.; Song, L.; Farshadfar, K.; Rudolph, M.; Rominger, F.; Oeser, T.; Ariafard, A.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2020, 59, 471.
doi: 10.1002/anie.v59.1 pmid: 29560212 |
|
|
(c) Zeng, Z.; Jin, H.; Sekine, K.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2018, 57, 6935.
pmid: 29560212 |
|
|
(d) Sahani, R. L.; Liu, R.-S. Angew. Chem., Int. Ed. 2017, 56, 12736.
doi: 10.1002/anie.v56.41 pmid: 29560212 |
|
|
(e) Sahani, R. L.; Liu, R.-S. Angew. Chem., Int. Ed. 2017, 56, 1026.
pmid: 29560212 |
|
|
(f) Shen, W.-B.; Xiao, X.-Y.; Sun, Q.; Zhou, B.; Zhu, X.-Q.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56, 605.
pmid: 29560212 |
|
|
(g) Zhou, A.-H.; He, Q.; Shu, C.; Yu, Y.-F.; Liu, S.; Zhao, T.; Zhang, W.; Lu, X.; Ye, L.-W. Chem. Sci. 2015, 6, 1265.
doi: 10.1039/c4sc02596b pmid: 29560212 |
|
| [6] |
For selected examples, see: (a) Garzón, M.; Davies, P. W. Org. Lett. 2014, 16, 4850.
pmid: 21351760 |
|
(b) Chatzopoulou, E.; Davies, P. W. Chem. Commun. 2013, 49, 8617.
pmid: 21351760 |
|
|
(c) Davies, P. W.; Cremonesi, A.; Dumitrescu, L. Angew. Chem., Int. Ed. 2011, 50, 8931.
pmid: 21351760 |
|
|
(d) Li, C.; Zhang, L. Org. Lett. 2011, 13, 1738.
doi: 10.1021/ol2002607 pmid: 21351760 |
|
| [7] |
For a review, see: Tian, X.; Song, L; Hashmi, A. S. K Chem.-Eur. J. 2020, 26, 3197.
pmid: 31793680 |
| [8] |
For selected examples, see: (a) Kawada, Y.; Ohmura, S.; Kobayashi, M.; Nojo, W.; Kondo, M.; Matsuda, Y.; Matsuoka, J.; Inuki, S.; Oishi, S.; Wang, C.; Saito, T.; Uchiyama, M.; Suzuki, T.; Ohno, H. Chem. Sci. 2018, 9, 8416.
pmid: 16089452 |
|
(b) Lonca, G. H.; Tejo, C.; Chan, H. L.; Chiba, S.; Gagosz, F. Chem. Commun. 2017, 53, 736.
pmid: 16089452 |
|
|
(c) Matsuoka, J.; Matsuda, Y.; Kawada, Y.; Oishi, S.; Ohno, H. Angew. Chem., Int. Ed. 2017, 56, 7444.
pmid: 16089452 |
|
|
(d) Yan, Z.-Y.; Xiao, Y.; Zhang, L. Angew. Chem., Int. Ed. 2012, 51, 8624.
pmid: 16089452 |
|
|
(e) Wetzel, A.; Gagosz, F. Angew. Chem.. Int. Ed. 2011, 50, 7354.
doi: 10.1002/anie.v50.32 pmid: 16089452 |
|
|
(f) Lu, B.; Luo, Y.; Liu, L.; Ye, L.; Wang, Y.; Zhang, L. Angew. Chem., Int. Ed. 2011, 50, 8358.
doi: 10.1002/anie.v50.36 pmid: 16089452 |
|
|
(g) Gorin, D. J.; Davis, N. R.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 11260.
pmid: 16089452 |
|
| [9] |
For recent reviews on ynamide reactivity, see: (a) Hong, F.-L.; Ye, L.-W. Acc. Chem. Res. 2020, 53, 2003.
pmid: 20429503 |
|
(b) Zhou, B.; Tan, T.-D.; Zhu, X.-Q.; Shang, M.; Ye, L.-W. ACS Catal. 2019, 9, 6393.
pmid: 20429503 |
|
|
(c) Pan, F.; Shu, C.; Ye, L.-W. Org. Biomol. Chem. 2016, 14, 9456.
pmid: 20429503 |
|
|
(d) Evano, G.; Theunissen, C.; Lecomte, M. Aldrichim. Acta 2015, 48, 59.
pmid: 20429503 |
|
|
(e) Wang, X.-N.; Yeom, H.-S.; Fang, L.-C.; He, S.; Ma, Z.-X.; Kedrowski, B. L.; Hsung, R. P. Acc. Chem. Res. 2014, 47, 560.
doi: 10.1021/ar400193g pmid: 20429503 |
|
|
(f) DeKorver, K. A.; Li, H.; Lohse, A. G.; Hayashi, R.; Lu, Z.; Zhang, Y.; Hsung, R. P. Chem. Rev. 2010, 110, 5064.
pmid: 20429503 |
|
|
(g) Evano, G.; Coste, A.; Jouvin, K. Angew. Chem., Int. Ed. 2010, 49, 2840.
pmid: 20429503 |
|
| [10] |
For selected examples by our group, see: (a) Liu, X.; Wang, Z.-S.; Zhai, T.-Y.; Luo, C.; Zhang, Y.-P.; Chen, Y.-B.; Deng, C.; Liu, R.-S.; Ye, L.-W. Angew. Chem., Int. Ed. 2020, 59, 17984.
doi: 10.1002/anie.v59.41 pmid: 29170497 |
|
(b) Hong, F.-L.; Chen, Y.-B.; Ye, S.-H.; Zhu, G.-Y.; Zhu, X.-Q.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142, 7618.
pmid: 29170497 |
|
|
(c) Wang, Z.-S.; Chen, Y.-B.; Zhang, H.-W.; Sun, Z.; Zhu, C.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142, 3636.
pmid: 29170497 |
|
|
(d) Huang, E.-H.; Zhang, Z.-X.; Ye, S.-H.; Chen, Y.-B.; Luo, W.-F.; Qian, P.-C.; Ye, L.-W. Chin. J. Chem. 2020, 38, 1086.
pmid: 29170497 |
|
|
(e) Li, H.-H.; Ye, S.-H.; Chen, Y.-B.; Luo, W.-F.; Qian, P.-C.; Ye, L.-W. Chin. J. Chem. 2020, 38, 263.
doi: 10.1002/cjoc.v38.3 pmid: 29170497 |
|
|
(f) Hong, F.-L.; Wang, Z.-S.; Wei, D.-D.; Zhai, T.-Y.; Deng, G.-C.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2019, 141, 16961.
doi: 10.1021/jacs.9b09303 pmid: 29170497 |
|
|
(g) Xu, Y.; Sun, Q.; Tan, T.-D.; Yang, M.-Y.; Yuan, P.; Wu, S.-Q.; Lu, X.; Hong, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2019, 58, 16252.
pmid: 29170497 |
|
|
(h) Zhou, B.; Zhang, Y.-Q.; Zhang, K.; Yang, M.-Y.; Chen, Y.-B.; Li, Y.; Peng, Q.; Zhu, S.-F.; Zhou, Q.-L.; Ye, L.-W. Nat. Commun. 2019, 10, 3234.
doi: 10.1038/s41467-019-11245-2 pmid: 29170497 |
|
|
(i) Li, L.; Zhu, X.-Q.; Zhang, Y.-Q.; Bu, H.-Z.; Yuan, P.; Chen, J.; Su, J.; Deng, X.; Ye, L.-W. Chem. Sci. 2019, 10, 3123.
pmid: 29170497 |
|
|
(j) Zheng, R.-H.; Guo, H.-C.; Yang, M.-Y.; Liu, M.-Q.; Ye, L.-W. Chin. J. Org. Chem. 2019, 39, 1672. (in Chinese)
pmid: 29170497 |
|
|
(郑人华, 郭海昌, 阳明洋, 刘梦琪, 叶龙武, 有机化学, 2019, 39, 1672.)
pmid: 29170497 |
|
|
(k) Zhu, J.; Ren, X.; Tang, F.; Pan, F.; Ye, L.-W. Chin. J. Org. Chem. 2019, 39, 1102. (in Chinese)
pmid: 29170497 |
|
|
(朱建荣, 任小娟, 唐飞宇, 潘飞, 叶龙武, 有机化学, 2019, 39, 1102.)
pmid: 29170497 |
|
|
(l) Zhou, B.; Li, L.; Zhu, X.-Q.; Yan, J.-Z.; Guo, Y.-L.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56, 4015.
doi: 10.1002/anie.201700596 pmid: 29170497 |
|
|
(m) Shen, W.-B.; Sun, Q.; Li, L.; Liu, X.; Zhou, B.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Nat. Commun. 2017, 8, 1748.
doi: 10.1038/s41467-017-01853-1 pmid: 29170497 |
|
|
(n) Li, L.; Chen, X.-M.; Wang, Z.-S.; Zhou, B.; Liu, X.; Lu, X.; Ye, L.-W. ACS Catal. 2017, 7, 4004.
pmid: 29170497 |
|
| [11] |
Feng, J.; Yi, X.; Fu, Y.; Yu, Y.; Huang, F. Chin. J. Org. Chem. 2019, 39, 3013. (in Chinese)
|
|
(封佳俊, 易享炎, 傅耀锋, 于杨, 黄菲, 有机化学, 2019, 39, 3013.)
|
|
| [12] |
Shu, C.; Wang, Y.-H.; Zhou, B.; Li, X.-L.; Ping, Y.-F.; Lu, X.; Ye, L.-W. J. Am. Chem. Soc. 2015, 137, 9567.
doi: 10.1021/jacs.5b06015 pmid: 26196678 |
| [13] |
Badigenchala, S.; Rajeshkumar, V.; Sekar, G. Org. Biomol. Chem. 2016, 14, 2297.
doi: 10.1039/c5ob02449h pmid: 26795352 |
| [1] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [2] | 宋晓, 卿晶, 黎君, 贾雪雷, 吴福松, 黄均荣, 金剑, 游恒志. 铜催化格氏试剂的不对称烯丙基烷基化连续流反应[J]. 有机化学, 2023, 43(9): 3174-3179. |
| [3] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [4] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [5] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [6] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [7] | 陆晓雨, 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅. 铜催化氟代丙烯酸与氧杂吖丙啶的脱羧交叉偶联反应[J]. 有机化学, 2023, 43(6): 2110-2119. |
| [8] | 鲍志成, 李慕尧, 王剑波. 铜催化芳基重氮乙酸酯与双[(频哪醇)硼基]甲烷的偶联反应[J]. 有机化学, 2023, 43(5): 1808-1814. |
| [9] | 李春生, 连晓琪, 陈莲芬. 铜催化亚砜叶立德与邻苯二胺[4+2]环加成反应[J]. 有机化学, 2023, 43(4): 1492-1498. |
| [10] | 刘洋, 黄翔, 王敏, 廖建. 铜催化环酮亚胺与β,γ-不饱和N-酰基吡唑不对称Mannich-Type反应[J]. 有机化学, 2023, 43(4): 1499-1509. |
| [11] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [12] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [13] | 刘春阳, 李燕, 张前. 铜催化环状烯烃烯丙位C(sp3)—H磺酰化反应研究[J]. 有机化学, 2023, 43(3): 1091-1101. |
| [14] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [15] | 韩彪, 李维双, 陈舒晗, 张泽浪, 赵雪, 张瑶瑶, 朱磊. 铜催化不饱和化合物硅加成反应的研究进展[J]. 有机化学, 2023, 43(2): 555-572. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||