有机化学 ›› 2021, Vol. 41 ›› Issue (12): 4639-4650.DOI: 10.6023/cjoc202107022 上一篇 下一篇
综述与进展
孟娜a, 刘启顺a, 刘瑞生a, 吕玉芬a,*( ), 赵晓辉b,*(
), 赵晓辉b,*( ), 魏伟a,b,*(
), 魏伟a,b,*( )
)
收稿日期:2021-07-07
修回日期:2021-07-29
发布日期:2021-08-09
通讯作者:
吕玉芬, 赵晓辉, 魏伟
作者简介:基金资助:
Na Menga, Qishun Liua, Ruisheng Liua, Yufen Lüa( ), Xiaohui Zhaob(
), Xiaohui Zhaob( ), Wei Weia,b(
), Wei Weia,b( )
)
Received:2021-07-07
Revised:2021-07-29
Published:2021-08-09
Contact:
Yufen Lü, Xiaohui Zhao, Wei Wei
About author:Supported by:文章分享

芳基和砜基化合物在合成化学、药物化学、材料化学等领域具有广泛的应用价值. 因此, 其清洁、高效的构建方法备受人们的关注. 芳基偶氮砜在光、电或热条件下可以发生C—N和S—N键均裂, 产生芳基和砜基自由基, 进而发生芳基化或砜基化反应选择性构建芳基或砜基化合物. 归纳总结了近年来芳基偶氮砜参与的芳基化及砜基化反应最新研究进展, 重点介绍了其合成方法及反应机理, 并对该领域的未来发展进行了展望.
孟娜, 刘启顺, 刘瑞生, 吕玉芬, 赵晓辉, 魏伟. 芳基偶氮砜的芳基化及砜基化研究进展[J]. 有机化学, 2021, 41(12): 4639-4650.
Na Meng, Qishun Liu, Ruisheng Liu, Yufen Lü, Xiaohui Zhao, Wei Wei. Recent Advances in Arylations and Sulfonylations of Arylazo Sulfones[J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4639-4650.
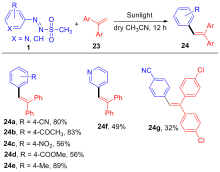

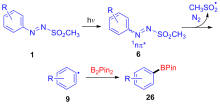
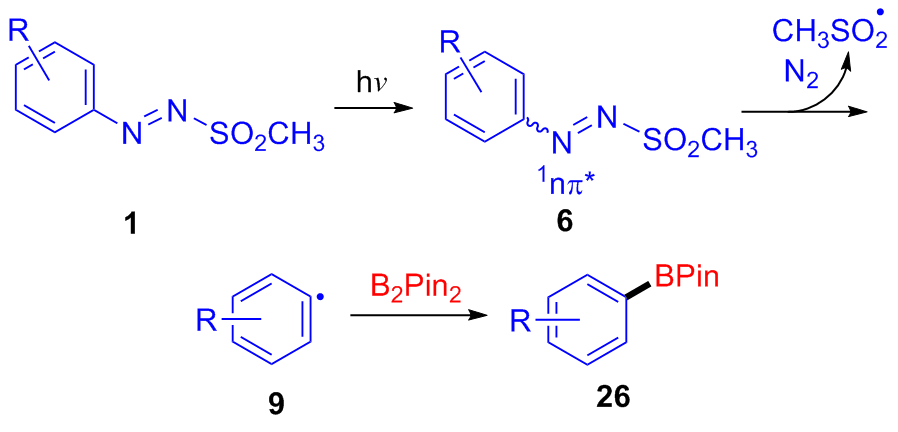
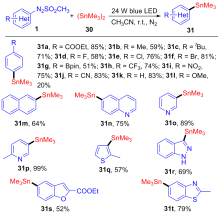
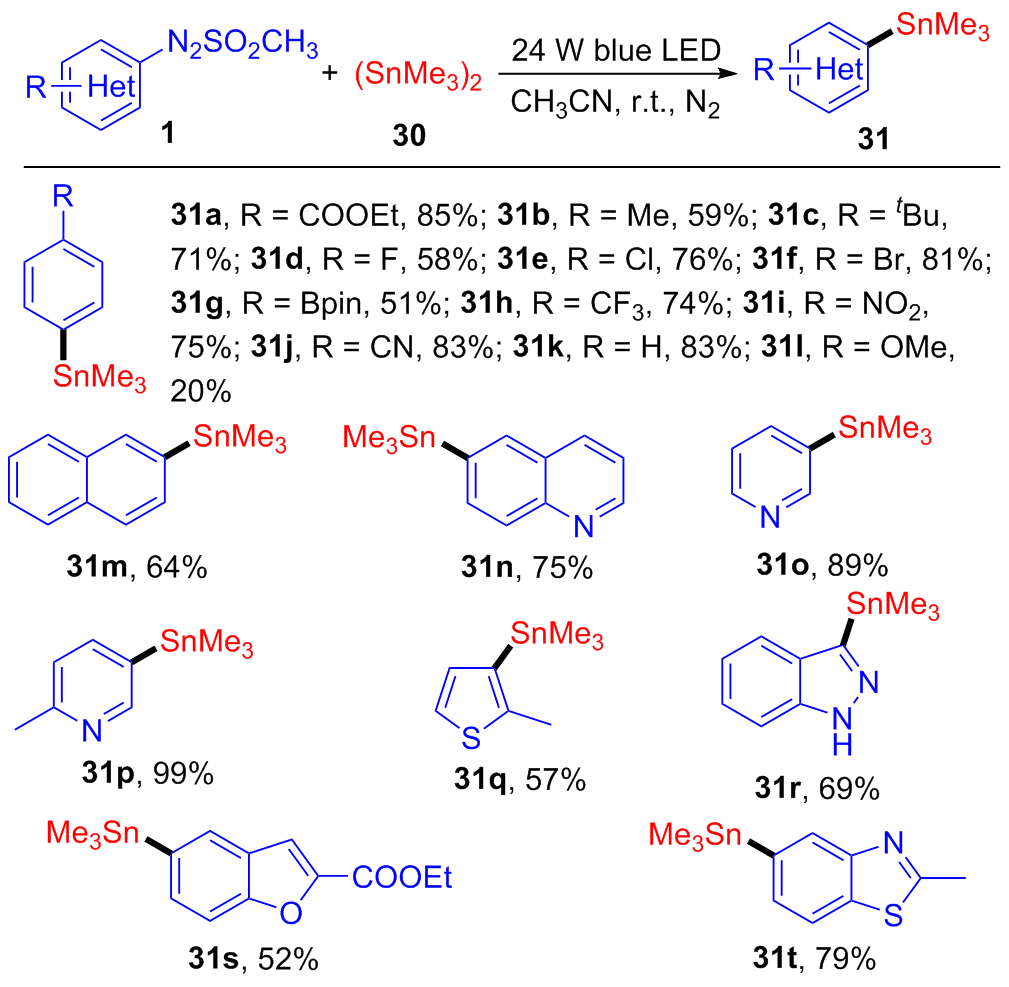
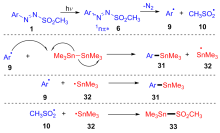
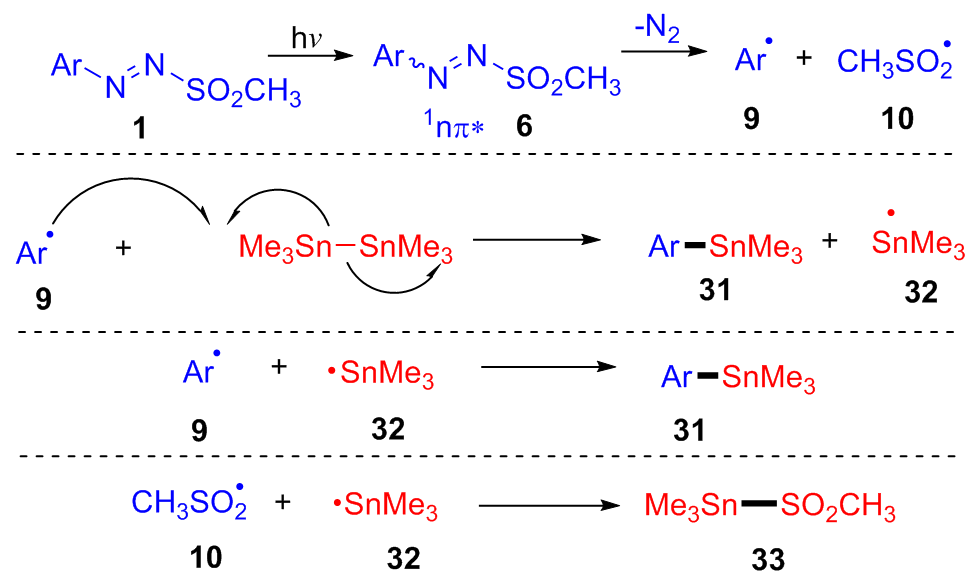
| [1] |
(a) Hanson, J. R. Nat. Prod. Rep. 1995, 12, 381.
pmid: 7666979 |
|
(b) Tietze, L. F.; Raschke, T. Synlett 1995, 597.
pmid: 7666979 |
|
| [2] |
(a) Shiotani, S.; Kometani, T.; Mitsuhashi, K.; Nozawa, T.; Kurobe, A.; Futsukaichi, O. J. Med. Chem. 1976, 19, 803.
pmid: 7674 |
|
(b) Smethurst, P. W. R.; Forrest, W. H.; Hayden, J. Br. J. Anaesth. 1971, 43, 1129.
doi: 10.1093/bja/43.12.1129 pmid: 7674 |
|
| [3] |
(a) Roll, D. M.; Scheuer, P. J.; Matsumoto, G. K.; Clardy, J. J. Am. Chem. Soc. 1983, 105, 6177.
doi: 10.1021/ja00357a049 pmid: 7503989 |
|
(b) Yano, H.; Nakanishi, S. J. Biol. Chem. 1993, 268, 25846.
pmid: 7503989 |
|
| [4] |
Yuan, Z. Z.; Dai, Q.; Qiao, L.; Zhao, Y. Y.; Zhang, H. M.; Li, X. F. J. Membr. Sci. 2017, 541, 465.
doi: 10.1016/j.memsci.2017.07.036 |
| [5] |
(a) Mack, D. J.; Njardarson, J. T. Angew. Chem., Int. Ed. 2013, 52, 1543.
doi: 10.1002/anie.201208412 |
|
(b) Wang, N. Z.; Saidhareddy, P.; Jiang, X. F. Nat. Prod. Rep. 2020, 37, 246.
doi: 10.1039/C8NP00093J |
|
| [6] |
Yazdanyar, S.; Boer, J.; Ingvarsson, G.; Szepietowski, J. C.; Jemec, G. B. E. Dermatology 2011, 222, 342.
doi: 10.1159/000329023 |
| [7] |
Li, P.; Hu, D. Y.; Xie, D. D.; Chen, J. X.; Jin, L. H.; Song, B. A. J. Agric. Food Chem. 2018, 66, 3093.
doi: 10.1021/acs.jafc.7b06061 |
| [8] |
(a) Williams, T. M.; Ciccarone, T. M.; MacTough, S. C.; Rooney, C. S.; Balani, S. K.; Condra, J. H.; Emini, E. A.; Goldman, M. E.; Greenlee, W. J. J. Med. Chem. 1993, 36, 1291.
pmid: 8558522 |
|
(b) Artico, M.; Silvestri, R.; Massa, S.; Loi, A. G.; Corrias, S.; Piras, G.; Colla, P. L. J. Med. Chem. 1996, 39, 522.
pmid: 8558522 |
|
| [9] |
Kamigta, N.; Kobayashi, M. Sulfur Rep. 1982, 2, 87.
|
| [10] |
(a) Kice, J. L.; Gabrielsen, R. S. J. Org. Chem. 1970, 35, 1004.
doi: 10.1021/jo00829a031 pmid: 32956584 |
|
(b) Kobayashi, M.; Gotoh, M.; Minato, H. J. Org. Chem. 1975, 40, 140.
doi: 10.1021/jo00889a043 pmid: 32956584 |
|
|
(c) Evers, M. J.; Christiaens, L. E.; Guillaume, M. R.; Renson, M. J. J. Org. Chem. 1985, 50, 1779.
doi: 10.1021/jo00210a050 pmid: 32956584 |
|
|
(d) Sapountzis, I.; Knochel, P. Angew. Chem., Int. Ed. 2004, 43, 897.
pmid: 32956584 |
|
|
(e) Qiu, D.; Lian, C.; Mao, J.; Fagnoni, M.; Protti, S. J. Org. Chem. 2020, 85, 12813.
doi: 10.1021/acs.joc.0c01895 pmid: 32956584 |
|
| [11] |
(a) Gaikwad, D. S.; Pore, D. M. Synlett 2012, 23, 2631.
doi: 10.1055/s-00000083 |
|
(b) Wang, L.; Bao, P.; Liu, W.; Liu, S.; Hu, C.; Yue, H.; Yang, D.; Wei, W. Chin. J. Org. Chem. 2018, 38, 3189. (in Chinese)
doi: 10.6023/cjoc201807014 |
|
|
( 王雷雷, 鲍鹏丽, 刘维伟, 刘思彤, 胡昌松, 岳会兰, 杨道山, 魏伟, 有机化学, 2018, 38, 3189.)
doi: 10.6023/cjoc201807014 |
|
| [12] |
(a) Shen, Q. L.; Ogata, T.; Hartwig, J. F. J. Am. Chem. Soc. 2008, 130, 6586.
doi: 10.1021/ja077074w |
|
(b) Arockiam, P. B.; Fischmeister, C.; Bruneau, C.; Dixneuf, P. H. Green Chem. 2013, 15, 67.
doi: 10.1039/C2GC36222H |
|
|
(c) Prades, A.; Poyatos, M.; Peris, E. Adv. Synth. Catal. 2010, 352, 1155.
doi: 10.1002/adsc.v352:7 |
|
| [13] |
(a) Rao Volla, C. M.; Vogel, P. Angew. Chem., Int. Ed. 2008, 47, 1305.
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Zeng, X. M.; Ilies, L.; Nakamura, E. J. Am. Chem. Soc. 2011, 133, 17638.
doi: 10.1021/ja209300c |
|
| [14] |
(a) Duong, H. A.; Gilligan, R. E.; Cooke, M. L.; Phipps, R. J.; Gaunt, M. J. Angew. Chem., Int. Ed. 2011, 50, 463.
doi: 10.1002/anie.201004704 |
|
(b) Vásquez-Céspedes, S.; Holtkamp, M.; Karst, U.; Glorius, F. Synlett 2017, 28, 2759.
doi: 10.1055/s-0036-1589007 |
|
| [15] |
(a) Kumar, M. R.; Park, K.; Lee, S. Adv. Synth. Catal. 2010, 352, 3255.
doi: 10.1002/adsc.v352.18 pmid: 12889906 |
|
(b) Hodgetts, K. J.; Kershaw, M. T. Org. Lett. 2003, 5, 2911.
pmid: 12889906 |
|
| [16] |
(a) Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Chem. Soc. Rev. 2016, 45, 2044.
doi: 10.1039/C5CS00655D |
|
(b) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506.
doi: 10.1021/acs.chemrev.0c00030 |
|
|
(c) He, W.-B.; Gao, L.-Q.; Chen, X.-J.; Wu, Z.-L.; Huang, Y.; Cao, Z.; Xu, X.-H.; He, W.-M. Chin. Chem. Lett. 2020, 31, 1895.
doi: 10.1016/j.cclet.2020.02.011 |
|
|
(d) Liu, Q.; Wang, L.; Yue, H.; Li, J.-S.; Luo, Z.; Wei, W. Green Chem. 2019, 21, 1609.
doi: 10.1039/C9GC00222G |
|
|
(e) Gan, Z.; Li, G.; Yang, X.; Yan, Q.; Xu, G.; Li, G.; Jiang, Y.-Y.; Yang, D. Sci. China: Chem. 2020, 63, 1652.
doi: 10.1007/s11426-020-9811-6 |
|
|
(f) Chen, J.-R.; Yan, D.-M.; Wei, Q.; Xiao, W.-J. ChemPhotoChem 2017, 1, 148.
doi: 10.1002/cptc.201700008 |
|
|
(g) Liu, R.; Liu, Q.; Meng, H.; Ding, H.; Hao, J.; Ji, Z.; Yue, H.; Wei, W. Org. Chem. Front. 2021, 8, 1970.
doi: 10.1039/D0QO01587C |
|
|
(h) Ye, H.; Xiao, C.; Lu, L. Chin. J. Org. Chem. 2018, 38, 1897. (in Chinese)
|
|
|
( 叶辉, 肖聪, 陆良秋, 有机化学, 2018, 38, 1897.)
doi: 10.6023/cjoc201804035 |
|
| [17] |
Crespi, S.; Protti, S.; Fagnoni, M. J. Org. Chem. 2016, 81, 9612.
doi: 10.1021/acs.joc.6b01619 |
| [18] |
Dossena, A.; Sampaolesi, S.; Palmieri, A.; Protti, S.; Fagnoni, M. J. Org. Chem. 2017, 82, 10687.
doi: 10.1021/acs.joc.7b01532 |
| [19] |
Sauer, C.; Liu, Y.; Nisi, A. D.; Protti, S.; Fagnoni, M.; Bandini, M. ChemCatChem 2017, 9, 4456.
doi: 10.1002/cctc.201701436 |
| [20] |
Malacarne, M.; Protti, S.; Fagnoni, M. Adv. Synth. Catal. 2017, 359, 3826.
doi: 10.1002/adsc.v359.21 |
| [21] |
Onuigbo, L.; Raviola, C.; Fonzo, A. D.; Protti, S.; Fagnoni, M. Eur. J. Org. Chem. 2018, 2018, 5297.
doi: 10.1002/ejoc.201800883 |
| [22] |
Xu, Y. L.; Yang, X. Y.; Fang, H. J. Org. Chem. 2018, 83, 12831.
doi: 10.1021/acs.joc.8b01662 |
| [23] |
(a) Lawrence, D. S.; Copper, J. E.; Smith, C. D. J. Med. Chem. 2001, 44, 594.
pmid: 15267248 |
|
(b) Willardsen, J. A.; Dudley, D. A.; Cody, W. L.; Chi, L.; McClanahan, T. B.; Mertz, T. E.; Potoczak, R. E.; Narasimhan, L. S.; Holland, D. R.; Rapundalo, S. T.; Edmunds, J. J. J. Med. Chem. 2004, 47, 4089.
pmid: 15267248 |
|
|
(c) Galal, S. A.; Khairat, S. H. M.; Ragab, F. A. F.; Abdelsamie, A. S.; Ali, M. M.; Soliman, S. M.; Mortier, J.; Wolber, G.; El Diwani, H. I. Eur. J. Med. Chem. 2014, 86, 122.
doi: 10.1016/j.ejmech.2014.08.048 pmid: 15267248 |
|
| [24] |
(a) Yuan, J.; Zhu, J.; Fu, J.; Yang, L.; Xiao, Y.; Mao, P.; Dua, X.; Qu, L. Org. Chem. Front. 2019, 6, 925.
doi: 10.1039/C9QO00063A pmid: 30372088 |
|
(b) Wei, W.; Wang, L.; Bao, P.; Shao, Y.; Yue, H.; Yang, D.; Yang, X.; Zhao, X.; Wang, H. Org. Lett. 2018, 20, 7125.
doi: 10.1021/acs.orglett.8b03079 pmid: 30372088 |
|
|
(d) Meng, N.; Lü, Y.; Liu, Q.; Liu, R.; Zhao, X.; Wei, W. Chin. Chem. Lett. 2021, 32, 258.
doi: 10.1016/j.cclet.2020.11.034 pmid: 30372088 |
|
|
(e) Bao, P.; Liu, F.; Lü, Y.; Yue, H.; Li, J.-S.; Wei, W. Org. Chem. Front. 2020, 7, 492.
doi: 10.1039/C9QO01334B pmid: 30372088 |
|
|
(h) Shi, J.; Wei, W. Chin. J. Org. Chem. 2020, 40, 2170. (in Chinese)
doi: 10.6023/cjoc202000041 pmid: 30372088 |
|
|
( 时建伟, 魏伟, 有机化学, 2020, 40, 2170.)
doi: 10.6023/cjoc202000041 pmid: 30372088 |
|
| [25] |
Jung, H. I.; Lee, J. H.; Kim, D. Y. Bull. Korean Chem. Soc. 2018, 39, 1003.
doi: 10.1002/bkcs.2018.39.issue-8 |
| [26] |
Lian, C.; Yue, G. L.; Mao, J. S.; Liu, D. Y.; Ding, Y.; Liu, Z. R.; Qiu, D.; Zhao, X.; Lu, K.; Fagnoni, M.; Protti, S. Org. Lett. 2019, 21, 5187.
doi: 10.1021/acs.orglett.9b01788 |
| [27] |
Qiu, D.; Lian, C.; Mao, J. S.; Ding, Y.; Liu, Z. R.; Wei, L. Y.; Fagnoni, M.; Protti, S. Adv. Synth. Catal. 2019, 361, 5239.
doi: 10.1002/adsc.201900953 |
| [28] |
Liu, Q. S.; Wang, L. L.; Yue, H. L.; Li, J. S.; Luo, Z. D.; Wei, W. Green Chem. 2019, 21, 1609.
doi: 10.1039/C9GC00222G |
| [29] |
(a) Zhang, L.; Niu, C.; Yang, X.; Qin, H.; Yang, J.; Wen, J.; Wang, H. Chin. J. Org. Chem. 2020, 40, 1117. (in Chinese)
doi: 10.6023/cjoc201912011 |
|
( 张龙菲, 牛聪, 杨晓婷, 秦宏云, 杨建静, 文江伟, 王桦, 有机化学, 2020, 40, 1117.)
doi: 10.6023/cjoc201912011 |
|
|
(b) Feng, E.; Hou, Z.; Xu, H. Chin. J. Org. Chem. 2019, 39, 1424. (in Chinese)
doi: 10.6023/cjoc201812007 |
|
|
( 冯恩祺, 侯中伟, 徐海超, 有机化学, 2019, 39, 1424.)
doi: 10.6023/cjoc201812007 |
|
|
(c) Li, M.; Wang, R.; Hao, W.; Jiang, B. Chin. J. Org. Chem. 2020, 40, 1540. (in Chinese)
doi: 10.6023/cjoc202002029 |
|
|
( 李梦帆, 王榕, 郝文娟, 姜波, 有机化学, 2019, 39, 1540.)
|
|
|
(d) Meng, W.; Xu, K.; Guo, B.; Zeng, C. Chin. J. Org. Chem. 2021, 41, 2621. (in Chinese)
doi: 10.6023/cjoc202102001 |
|
|
( 孟薇, 徐坤, 郭兵兵, 曾程初, 有机化学, 2021, 41, 2621.)
doi: 10.6023/cjoc202102001 |
|
|
(e) Wu, Y.; Chen, J.-Y.; Ning, J.; Jiang, X.; Deng, J.; Deng, Y.; Xu, R.; He, W.-M. Green Chem. 2021, 23, 3950.
doi: 10.1039/D1GC00562F |
|
|
(f) Chen, J.-Y.; Zhong, C.-T.; Gui, Q.-W.; Zhou, Y.-M.; Fang, Y.-Y.; Liu, K.-J.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 475.
doi: 10.1016/j.cclet.2020.09.034 |
|
| [30] |
Wang, R. K.; Chen, F. M.; Jiang, L. Q.; Yi, W. B. Adv. Synth. Catal. 2020, 363, 1904.
doi: 10.1002/adsc.v363.7 |
| [31] |
(a) Li, Y.; Wan, J.-P. Chin. J. Org. Chem. 2020, 40, 3889. (in Chinese)
doi: 10.6023/cjoc202005026 |
|
( 李毅, 万结平, 有机化学, 2020, 40, 3889.)
doi: 10.6023/cjoc202005026 |
|
|
(b) Liu, Q.; Lü, Y.; Liu, R.; Zhao, X.; Wang, J.; Wei, W. Chin. Chem. Lett. 2021, 32, 136.
doi: 10.1016/j.cclet.2020.11.059 |
|
|
(c) Bao, W.-H.; Wang, Z.; Tang, X.; Zhang, Y.-F.; Tan, J.-X.; Zhu, Q.; Cao, Z.; Lin, Y.-W.; He, W.-M. Chin. Chem. Lett. 2019, 30, 2259.
doi: 10.1016/j.cclet.2019.06.052 |
|
|
(d) Lia, G.-H.; Han, Q.-Q.; Sun, Y.-Y.; Chen, D.-M.; Wang, Z.-L.; Xu, X.-M.; Yu, X.-Y. Chin. Chem. Lett. 2020, 31, 3255.
doi: 10.1016/j.cclet.2020.03.007 |
|
|
(e) Wang, L.; Zhang, M.; Zhang, Y.; Liu, Q.; Zhao, X.; Li, J.-S.; Luo, Z.; Wei, W. Chin. Chem. Lett. 2020, 31, 67.
doi: 10.1016/j.cclet.2019.05.041 |
|
| [32] |
Wei, W.; Liu, C.; Yang, D.; Wen, J.; You, J.; Suo, Y.; Wang, H. Chem. Commun. 2013, 49, 10239.
doi: 10.1039/c3cc45803b |
| [33] |
Liu, Q. S.; Liu, F.; Yue, H. L.; Zhao, X. H.; Li, J. S.; Wei, W. Adv. Synth. Catal. 2019, 361, 5277.
doi: 10.1002/adsc.v361.22 |
| [34] |
Lü, Y. F.; Liu, Q. S.; Liu, F.; Yue, H. L.; Li, J. S.; Wei, W. Tetrahedron Lett. 2020, 61, 151335.
doi: 10.1016/j.tetlet.2019.151335 |
| [35] |
Chawla, R.; Jaiswal, S.; Dutta, P. K.; Yadav, L. D. S. Tetrahedron Lett. 2020, 61, 151898.
doi: 10.1016/j.tetlet.2020.151898 |
| [36] |
Liu, Q. S.; Lü, Y. F.; Liu, R. S.; Zhao, X. H.; Wang, J. W.; Wei, W. Chin. Chem. Lett. 2021, 32, 136.
doi: 10.1016/j.cclet.2020.11.059 |
| [1] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [2] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [3] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [4] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [5] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [6] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [7] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [8] | 刘颖杰, 石岗庆, 仇格, 张鑫, 宋冬雪, 陈宁, 于淼, 许颖. 光/电催化醚α-位官能团化研究进展[J]. 有机化学, 2023, 43(8): 2664-2681. |
| [9] | 杨晓娜, 郭宏宇, 周荣. 可见光促进有机硅化合物参与的化学转化[J]. 有机化学, 2023, 43(8): 2720-2742. |
| [10] | 普佳霞, 贾小英, 韩丽荣, 李清寒. 可见光诱导C—N键断裂构建C—C键的研究进展[J]. 有机化学, 2023, 43(8): 2591-2613. |
| [11] | 王灵娜, 刘晓庆, 林钢, 金泓颖, 焦民均, 刘雪粉, 罗书平. 光促进双(4-二苯甲酮)苯醚催化C(sp3)—H键活化构建C—S键[J]. 有机化学, 2023, 43(8): 2848-2854. |
| [12] | 赵瑜, 张凯, 白育斌, 张琰图, 史时辉. 无金属条件下可见光催化与溴盐协同促进烯烃的氢硅化反应研究[J]. 有机化学, 2023, 43(8): 2837-2847. |
| [13] | 徐忠荣, 万结平, 刘云云. 基于热、光以及电化学过程的无过渡金属碳-氢键硫氰化和硒氰化反应[J]. 有机化学, 2023, 43(7): 2425-2446. |
| [14] | 高艳华, 张银潘, 张妍, 宋涛, 杨勇. 可见光驱动表面富含氧空位Nb2O5催化醇氧化反应[J]. 有机化学, 2023, 43(7): 2572-2579. |
| [15] | 刘亚鑫, 张渔, 罗书平. 热延迟荧光(TADF)光敏剂的设计合成及其光催化脱卤反应性能研究[J]. 有机化学, 2023, 43(7): 2476-2483. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||