[1] (a) Özden S.; Atabey D.; Yildiz S.; Göker, H. Bioorg. Med. Chem.2005, 13, 1587.
(b) Froidevaux V.; Negrell C.; Caillol S.; Pascault J. P.; Boutevin B. Chem. Rev.2016, 116, 14181.
(c) Irrgang T.; Kempe R. Chem. Rev.2019, 119, 2524.
(d) Krska S. W.; DiRocco D. A.; Dreher S. D.; Shevlin, M. Acc. Chem. Res.2017, 50, 2976.
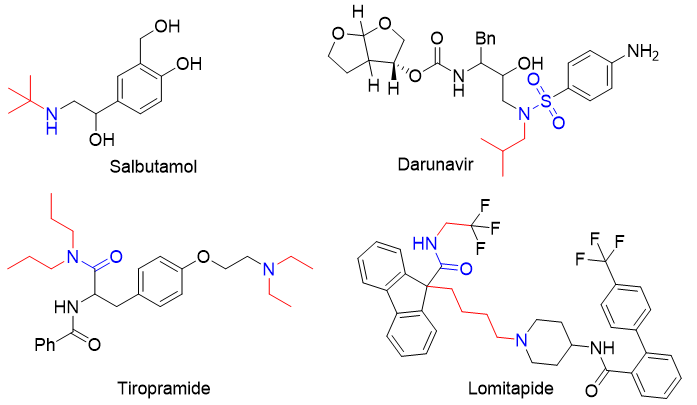
[2] Mellick L.; Walsh P.; Clanton C.; Kalra S.; McKinney S. Pediatr. Pulmonol.2025, 60, e27391.
[3] Kitaw T. A.; Abate B. B.; Yilak G.; Tilahun B. D.; Faris A. M.; Walle G. T.; Haile, R. N. AIDS Res. Ther.2024, 21, 43.
[4] Sinagra E.; Morreale G. C.; Mohammadian G.; Fusco G.; Guarnotta V.; Tomasello G.; Cappello F.; Rossi F.; Amvrosiadis G.; Raimondo, D. World J. Gastroenterol.2017, 23, 6593.
[5] Nasser M.; Mohammed H. E.; Haseeb M. E.; Darwish M. K.; Hanen G.; Abdelaziz N. A.; Heiba A. H.; Shata A.; Abdelkader, M. S. Cardiovasc. Drugs Ther.2025, https://doi.org/10.1007/s10557-025-07764-4.
[6] de Figueiredo R. M.; Suppo J.-S.; Campagne J.-M. Chem. Rev.2016, 116, 12029.
[7] Liu W.; Chen J.; Su W. Pharm. Fronts2024, 6, e355.
[8] Hartwig, J. F. Acc. Chem. Res.2008, 41, 1534.
[9] Klinkenberg J. L.; Hartwig, J. F. Angew. Chem., Int. Ed.2011, 50, 86.
[10] Green R. A.; Hartwig, J. F. Org. Lett.2014, 16, 4388.
[11] Green R. A.; Hartwig, J. F. Angew. Chem., Int. Ed.2015, 54, 3768.
[12] (a) Guram A. S.; Rennels R. A.; Buchwald, S. L. Angew. Chem., Int. Ed.1995, 34, 1348.
(b) Anand, M.; Nørskov, J. K. ACS Catal. 2019, 10, 336.
(c) Wambua V.; Hirschi J. S.; Vetticatt, M. J. ACS Catal.2021, 11, 60.
(d) Schmidt O. P.; Blackmond, D. G. ACS Catal.2020, 10, 8926.
[13] Ruiz-Castillo P.; Buchwald, S. L. Chem. Rev.2016, 116, 12564.
[14] (a) Batey, R. A. In Boronic Acids, Eds.:Hall, D. G., Wiley-VCH, Weinheim, Germany, 2005, p. 279.
(b) Partyka, D. V.Chem. Rev. 2011, 111, 1529.
[15] (a) Ma, D.-W.; Zhang, Y.-D.; Yao, J.-C.; Wu, S.-H.; Tao, F.-G. J. Am. Chem. Soc. 1998, 120, 12459.
(b) Yang Q.; Zhao Y.-S.; Ma, D.-W. Org. Process Res. Dev.2022, 26, 1690.
16 (c) Kamal A.; Srinivasulu V.; Seshadri B. N.; Markandeya N.; Alarifi A.; Shankaraiah N. Green Chem.2012, 14, 2513.
[16] Irrgang T.; Kempe R. Chem. Rev.2020, 120, 9583.
[17] Murugesan K.; Senthamarai T.; Chandrashekhar V. G.; Natte K.; Kamer P. C. J.; Beller M.; Jagadeesh, R. V. Chem. Soc. Rev.2020, 49, 6273.
[18] (a) Arrayás, R. G.; Carretero, J. C.Chem. Soc. Rev. 2009, 38, 1940.
(b) Noble A.; Anderson, J. C. Chem. Rev.2013, 113, 2887.
[19] Ma S.; Hartwig, J. F. Acc. Chem. Res.2023, 56, 1565.
[20] Colonna P.; Bezzenine S.; Gil R.; Hannedouche, J. Adv. Synth. Catal.2020, 362, 1550.
[21] Erdik E.; Ay M.Chem. Rev. 1989, 89, 1947.
[22] Ciganek, E. Org. React. 2009, 72, 1.
[23] Trowbridge A.; Walton S. M.; Gaunt, M. J. Chem. Rev.2020, 120, 2613.
[24] Emadi R.; Nekoo B. A.; Molaverdi F.; Khorsandi Z.; Sheibani R.; Sadeghi-Aliabadi H. RSC Adv.2023, 13, 18715.
[25] Melchiorre, P. Chem. Rev. 2022, 122, 1483.
[26] Cheung K. P. S.; Sarkar S.; Gevorgyan V. Chem. Rev.2022, 122, 1543.
[27] Chan A. Y.; Perry I. B.; Bissonnette N. B.; Buksh B. F.; Edwards G. A.; Frye L. I.; Garry O. L.; Lavagnino M. N.; Li B. X.; Liang Y.; Mao E.; Millet A.; Oakley J. V.; Reed N. L.; Sakai H. A.; Seath C. P.; MacMillan, D. W. C. Chem. Rev.2022, 122, 1485.
[28] Escorihuela J.; Lledós A.; Ujaque G. Chem. Rev. 2023, 123, 9139.
[29] Rivas M.; Palchykov V.; Jia X.; Gevorgyan, V. Nat. Rev. Chem.2022, 6, 544.
[30] Chen J.-R.; Hu X.-Q.; Lu L.-Q.; Xiao W.-J.Chem. Soc. Rev. 2016, 45, 2044.
[31] Li S.; You L.; Li Y.; Shu, W. Chin. J. Org. Chem.2025, 45, 1460.
[32] Tian L.; Zhang H.-R.; Li Y.-L.; Ren Y.-F.; Shu, W. J. Org. Chem.2025, 90, 12833.
[33] Musacchio A. J.; Lainhart B. C.; Zhang X.; Naguib S. G.; Sherwood T. C.; Knowles R. R. Science2017, 355, 727.
[34] Miller D. C.; Ganley J. M.; Musacchio A. J.; Sherwood T. C.; Ewing W. R.; Knowles, R. R. J. Am. Chem. Soc.2019, 141, 16590.
[35] Du Y.-D.; Chen B.-H.; Shu, W. Angew. Chem., Int. Ed.2021, 60, 9875.
[36] Geunes E. P.; Meinhardt J. M.; Wu E. J.; Knowles, R. R. J. Am. Chem. Soc.2023, 145, 21738.
[37] Sedillo K.; Fan F.; Knowles R. R.; Doyle, A. G. J. Am. Chem. Soc.2024, 146, 20349.
[38] Miller D. C.; Choi G. J.; Orbe H. S.; Knowles, R. R. J. Am. Chem. Soc.2015, 137, 13492.
[39] Davies J.; Svejstrup T. D.; Reina D. F.; Sheikh N. S.; Leonori, D. J. Am. Chem. Soc.2016, 138, 8092.
[40] Ruccolo S.; Qin Y.; Schnedermann C.; Nocera, D. G. J. Am. Chem. Soc.2018, 140, 14926.
[41] Nguyen S. T.; Zhu Q.; Knowles, R. R. ACS Catal.2019, 9, 4502.
[42] Nguyen T. M.; Manohar N.; Nicewicz, D. A. Angew. Chem., Int. Ed.2014, 53, 6198.
[43] Zhu Q.; Graff D. E.; Knowles, R. R. J. Am. Chem. Soc.2018, 140, 741.
[44] Chinn A. J.; Sedillo K.; Doyle, A. G. J. Am. Chem. Soc.2021, 143, 18331.
[45] Lin A.; Karrasch M. J.; Yan Q.; Ganley J. M.; Hejna B. G.; Knowles, R. R. ACS Catal.2024, 14, 13098.
[46] Nguyen T. M.; Nicewicz, D. A. J. Am. Chem. Soc.2013, 135, 9588.
[47] Hu X.-Q.; Chen J.-R.; Wei Q.; Liu F.-L.; Deng Q.-H.; Beauchemin A. M.; Xiao, W.-J. Angew. Chem., Int. Ed.2014, 53, 12163.
[48] Chen J.; Guo H.-M.; Zhao Q.-Q.; Chen J.-R.; Xiao W.-J. Chem. Commun.2018, 54, 6780. |