有机化学 ›› 2019, Vol. 39 ›› Issue (11): 3190-3198.DOI: 10.6023/cjoc201904070 上一篇 下一篇
所属专题: 元素有机化学合辑2018-2019
研究论文
董道青a, 陈文静a, 陈德茂a, 李丽霞a, 李光辉a, 王祖利a*( ), 邓企b, 龙姝c
), 邓企b, 龙姝c
收稿日期:2019-04-29
发布日期:2019-07-03
通讯作者:
王祖利
E-mail:wangzulichem@163.com
基金资助:
Dong Daoqinga, Chen Wenjinga, Chen Demaoa, Li Lixiaa, Li Guanghuia, Wang Zulia*( ), Deng Qib, Long Shuc
), Deng Qib, Long Shuc
Received:2019-04-29
Published:2019-07-03
Contact:
Wang Zuli
E-mail:wangzulichem@163.com
Supported by:文章分享

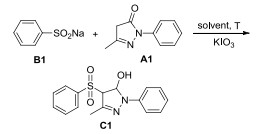
A facile and efficient method for the synthesis of sulfonated or sulfenylated pyrazolones catalyzed by KIO3 was established. A variety of desired products were obtained in moderate to high yields. This methodology could be conducted under mild reaction conditions without requiring any metal. Control experiments showed that the mechanism of this reaction was different from previous KIO3-catalyzed reactions. Some of these desired products showed high inhibitory activity against V. mali and B. cinerea.
董道青, 陈文静, 陈德茂, 李丽霞, 李光辉, 王祖利, 邓企, 龙姝. KIO3促进的直接合成硫化或磺酰化吡唑啉酮及其抗菌活性[J]. 有机化学, 2019, 39(11): 3190-3198.
Dong Daoqing, Chen Wenjing, Chen Demao, Li Lixia, Li Guanghui, Wang Zuli, Deng Qi, Long Shu. Direct Synthesis of Sulfonated or Sulfenylated Pyrazolones Mediated by KIO3 and Their Anti-microbial Activity[J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3190-3198.
 | ||||
| Entry | Catalyst (equiv.) | Solvent | T/℃ | Yield/% |
| 1 | KIO3 (0.30) | DMSO | 100 | 35 |
| 2 | KIO3 (0.30) | 1, 2-Dimethoxyethane | 100 | 40 |
| 3 | KIO3 (0.30) | EtOH | 100 | 62 |
| 4 | KIO3 (0.30) | THF | 100 | 24 |
| 5 | KIO3 (0.30) | Ethylene glycol | 100 | 48 |
| 6 | KIO3 (0.30) | CH3CN | 100 | Trace |
| 7 | KIO3 (0.30) | CHCl3 | 100 | Trace |
| 8 | KIO3 (0.30) | 1, 2-Dichloroethane | 100 | Trace |
| 9 | KIO3 (0.30) | 1, 4-Dioxane | 100 | Trace |
| 10 | KIO3 (0.30) | Toluene | 100 | Trace |
| 11 | KIO3 (0.30) | EtOH | 120 | 86 |
| 12 | KIO3 (0.30) | EtOH | 130 | 86 |
| 13 | KIO3 (0.20) | EtOH | 120 | 78 |
| 14b | — | EtOH | 120 | NR |
| 15 | KCl | EtOH | 120 | NR |
| 16c | KIO3 (0.30) | EtOH | 120 | 83 |
 | ||||
| Entry | Catalyst (equiv.) | Solvent | T/℃ | Yield/% |
| 1 | KIO3 (0.30) | DMSO | 100 | 35 |
| 2 | KIO3 (0.30) | 1, 2-Dimethoxyethane | 100 | 40 |
| 3 | KIO3 (0.30) | EtOH | 100 | 62 |
| 4 | KIO3 (0.30) | THF | 100 | 24 |
| 5 | KIO3 (0.30) | Ethylene glycol | 100 | 48 |
| 6 | KIO3 (0.30) | CH3CN | 100 | Trace |
| 7 | KIO3 (0.30) | CHCl3 | 100 | Trace |
| 8 | KIO3 (0.30) | 1, 2-Dichloroethane | 100 | Trace |
| 9 | KIO3 (0.30) | 1, 4-Dioxane | 100 | Trace |
| 10 | KIO3 (0.30) | Toluene | 100 | Trace |
| 11 | KIO3 (0.30) | EtOH | 120 | 86 |
| 12 | KIO3 (0.30) | EtOH | 130 | 86 |
| 13 | KIO3 (0.20) | EtOH | 120 | 78 |
| 14b | — | EtOH | 120 | NR |
| 15 | KCl | EtOH | 120 | NR |
| 16c | KIO3 (0.30) | EtOH | 120 | 83 |
| Compd. | V. mali | B. cinerea | C. glecosporioides |
| C4 | 82.63 | 78.15 | 25.81 |
| E2 | 81.38 | 58.82 | 24.26 |
| E12 | 81.12 | 66.39 | 50.52 |
| E14 | 83.33 | 56.17 | 39.69 |
| E16 | 81.67 | 34.40 | 38.92 |
| E15 | 70.34 | 38.25 | 26.29 |
| E11 | 75.34 | 52.9 | 20.73 |
| E3 | 57.66 | 56.15 | 11.11 |
| Compd. | V. mali | B. cinerea | C. glecosporioides |
| C4 | 82.63 | 78.15 | 25.81 |
| E2 | 81.38 | 58.82 | 24.26 |
| E12 | 81.12 | 66.39 | 50.52 |
| E14 | 83.33 | 56.17 | 39.69 |
| E16 | 81.67 | 34.40 | 38.92 |
| E15 | 70.34 | 38.25 | 26.29 |
| E11 | 75.34 | 52.9 | 20.73 |
| E3 | 57.66 | 56.15 | 11.11 |
| [1] |
(a) Elguero, J. Comprehensive Heterocyclic Chemistry, Eds.: Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Pergamon, Oxford, UK, 1996, p. 167.
doi: 10.1021/jm701372r |
|
(b) Liu, X. H.; Cui, P.; Song, B. A.; Bhadury, P. S.; Zhu, H. L.; Wang, S. F. Bioorg. Med. Chem. 2008, 16, 4075.
doi: 10.1021/jm701372r |
|
|
(c) Velaparthi, S.; Brunsteiner, M.; Uddin, R.; Wan, B.; Franzblau, S. G.; Petukhov, P. A. J. Med. Chem. 2008, 51, 1999.
doi: 10.1021/jm701372r |
|
|
(d) Magedov, I. V.; Manpadi, M.; Slambrouck, V. S.; Steelant, W. F. A.; Rozhkova, E.; Przheval'skii, N. M.; Rogelj, S.; Kornienko, A. J. Med. Chem. 2007, 50, 5183.
doi: 10.1021/jm701372r |
|
|
(e) Vicentini, C. B.; Romagnoli, C.; Reotti, E.; Mares, D. J. Agric. Food Chem. 2007, 55, 10331.
doi: 10.1021/jm701372r |
|
|
(f) Vicentini, C. B.; Guccione, S.; Giurato, L.; Ciaccio, R.; Mares, D.; Forlani, G. J. Agric. Food Chem. 2005, 53, 3848.
doi: 10.1021/jm701372r |
|
|
(g) Peng, L.; Hu, Z.; Tang, Z.; Jiao, Y.; Xu, X. Chin. Chem. Lett. 2019, 30, 1481.
doi: 10.1021/jm701372r |
|
|
(h) Liang, Q.; Zhang, Y.; Zeng, M.; Guan, L.; Xiao, Y.; Xiao, F. Toxicol. Res. 2018, 7, 521.
doi: 10.1021/jm701372r |
|
|
(i) Jiang, H.; Tang, X.; Xu, Z.; Wang, H.; Han, K.; Yang, X.; Zhou, Y.; Feng, Y.; Yu, X.; Gui, Q. Org. Biomol. Chem. 2019, 17, 2715.
doi: 10.1021/jm701372r |
|
|
(j) Li, G.; Gan, Z.; Kong, K.; Dou, X.; Yang, D. Adv. Synth. Catal. 2019, 361, 1808.
doi: 10.1021/jm701372r |
|
| [2] |
Kawakubo K. Shindo M. Konotsune T. Plant Physiol. 1979 64 774.
doi: 10.1104/pp.64.5.774 |
| [3] |
Watanabe K. Morinaka Y. Iseki K. Watanabe T. Yuki S. Nishi H. Redox Rep. 2003 8 151.
doi: 10.1179/135100003225001520 |
| [4] |
(a) Wolf, W. M. J. Mol. Struct. 1999, 474, 113.
doi: 10.1016/S0022-2860(98)00565-1 |
|
(b) Petrov, K. G.; Zhang, Y.; Carter, M.; Cockerill, G. S.; Dickerson, S.; Gauthier, C. A.; Guo, Y.; Mook, R. A.; Rusnak, D. W.; Walker, A. L.; Wood, E. R.; Lackey, K. E. Bioorg. Med. Chem. Lett. 2006, 16, 4686.
doi: 10.1016/S0022-2860(98)00565-1 |
|
|
(c) Ettari, R.; Nizi, E.; Francesco, M. E. D.; Dude, M. A.; Pradel, G.; Vicik, R.; Schirmeister, T.; Micale, N.; Grasso, S.; Zappala, M. J. Med. Chem. 2008, 51, 988.
doi: 10.1016/S0022-2860(98)00565-1 |
|
| [5] |
(a) Kumar, R.; Namboothiri, I. N. N. Org. Lett. 2011, 13, 4016.
doi: 10.1021/ol201534f |
|
(b) Kumar, R.; Verma, D.; Mobin, S. M.; Namboothiri, I. N. N. Org. Lett. 2012, 14, 4070.
doi: 10.1021/ol201534f |
|
|
(c) Zhu, Y.; Lu, W. T.; Sun, H. C.; Zhan, Z. P. Org. Lett. 2013, 15, 4146.
doi: 10.1021/ol201534f |
|
|
(d) Jeon, D. J.; Lee, J. N.; Lee, K. C.; Kim, H. R.; Zong, K.; Ryu, E. K. Bull. Korean Chem. Soc. 1998, 19, 1153.
doi: 10.1021/ol201534f |
|
|
(e) Xu, X. H.; Wang, X.; Liu, G. K.; Tokunaga, E.; Shibata, N. Org. Lett. 2012, 14, 2544.
doi: 10.1021/ol201534f |
|
| [6] |
Wei W. Cui H. Yang D. Liu X. He C. Dai S. Wang H. Org. Chem. Front. 2017 4 26.
doi: 10.1039/C6QO00403B |
| [7] |
(a) Shermolovich, Y. G.; Emets, S. V. Chem. Heterocycl. Compd. 2000, 36, 152.
doi: 10.1007/BF02283543 |
|
(b) Li, L.; Hao, S.; Dong, D.; Wang, Z. Tetrahedron. Lett. 2018, 59, 1517.
doi: 10.1007/BF02283543 |
|
| [8] |
Zhao X. Zhang L. Li T. Liu G. Wang H. Lu K. Chem. Commun. 2014 50 13121.
doi: 10.1039/C4CC05237D |
| [9] |
Wang D. Guo S. Zhang R. Lin S. Yan Z. RSC Adv. 2016 6 54377.
doi: 10.1039/C6RA02302A |
| [10] |
Liu X. Cui H. Yang D. Dai S. Zhang T. Sun J. Wei W. Wang H. RSC Adv. 2016 6 51830.
doi: 10.1039/C6RA09739A |
| [11] |
Yang D. Sun P. Wei W. Meng L. He L. Fang B. Jiang W. Wang H. Org. Chem. Front. 2016 3 1457.
doi: 10.1039/C6QO00407E |
| [12] |
Hu F. Gao W. Chang H. Li X. Wei W. Chin. J. Org. Chem. 2015 35 1848.
doi: 10.6023/cjoc201504039 |
| [13] |
(a) Mphahlele, M. J. Molecules 2009, 14, 4814.
doi: 10.3390/molecules14124814 |
|
(b) Hummel, S.; Kirsch, S. F. Beilstein J. Org. Chem. 2011, 7, 847.
doi: 10.3390/molecules14124814 |
|
| [14] |
(a) Zhao, J.; Gao, W.; Chang, H.; Li, X.; Liu, Q.; Wei, W. Chin. J. Org. Chem. 2014, 34, 1941.
doi: 10.6023/cjoc201405003 |
|
(b) Wu, C.; Lu, L. H.; Peng, A. Z.; Jia, G. K.; Peng, C.; Cao, Z.; Tang, Z.; He, W. M.; Xu, X. Green Chem. 2018, 20, 3683.
doi: 10.6023/cjoc201405003 |
|
|
(c) Bao, W. H.; He, M.; Wang, J.; Peng, T. X.; Sung, M.; Tang, Z.; Jiang, S.; Cao, Z.; He, W. M. J. Org. Chem. 2019, 84, 6065.
doi: 10.6023/cjoc201405003 |
|
|
(d) Lu, L.-H.; Wang, Z.; Xia, W.; Cheng, P.; Zhang, B.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2019, 30, 1237.
doi: 10.6023/cjoc201405003 |
|
| [15] |
(a) Liu, K.; Song, C.; Lei, A. Org. Biomol. Chem. 2018, 16, 2375.
doi: 10.6023/cjoc201810002 |
|
(b) Nguyen, T. B. Asian J. Org. Chem. 2017, 6, 477.
doi: 10.6023/cjoc201810002 |
|
|
(c) Lauriers, A. J.; Legault. C. Y. Asian J. Org. Chem. 2016, 5, 1078.
doi: 10.6023/cjoc201810002 |
|
|
(d) Yoshimura, A.; Yusubov, M. S.; Zhdankin. V. V. Org. Biomol. Chem. 2016, 21, 4771.
doi: 10.6023/cjoc201810002 |
|
|
(e) Aggarwal, T.; Kumar, S.; Verma, A. K. Org. Biomol. Chem. 2016, 14, 7639.
doi: 10.6023/cjoc201810002 |
|
|
(f) Zhao, J.; Gao, W.; Chang, H.; Li, X.; Liu, Q.; Wei, W. Chin. J. Org. Chem. 2014, 34, 1941.
doi: 10.6023/cjoc201810002 |
|
|
(g) Finkbeiner, P.; Nachtsheim. B. J. Synthesis 2013, 45, 979.
doi: 10.6023/cjoc201810002 |
|
|
(h) Liu M.; Huang H.; Chen Y. Chin. J. Chem. 2018, 36, 1209.
doi: 10.6023/cjoc201810002 |
|
|
(i) Wan, J.; Zhong, S.; Guo, Y.; Wei, L. Eur. J. Org. Chem. 2017, 4401.
doi: 10.6023/cjoc201810002 |
|
|
(j) Hou J.; Zhang X.; Yu W.; Chang J. Chin. J. Org. Chem. 2018, 38, 3236.
doi: 10.6023/cjoc201810002 |
|
|
(k) Lu, L. H.; Zhou, S. J.; He, W. B.; Xia, W.; Chen, P.; Yu, X.; Xu, X.; He, W. M. Org. Biomol. Chem. 2018, 16, 9064.
doi: 10.6023/cjoc201810002 |
|
|
(l) Bao, W. H.; Wu, C.; Wang, J. T.; Xia, W.; Chen, P.; Tang, Z.; Xu, X., He, W. M. Org. Biomol. Chem. 2018, 16, 8403.
doi: 10.6023/cjoc201810002 |
|
|
(m) Lu, L. H.; Zhou, S. J.; Sun, M.; Chen, J. L.; Xia, W.; Yu, X.; Xu, X.; He, W. M. ACS Sustainable Chem. Eng. 2019, 7, 1574.
doi: 10.6023/cjoc201810002 |
|
|
(n) Tang, Y. Ran, S.; Wang, P.; Chen, P. Chin. J. Org. Chem. 2019, 39, 1116 (in Chinese).
doi: 10.6023/cjoc201810002 |
|
|
(唐裕才, 冉书童, 王萍, 陈飘, 有机化学, 2019, 39, 1116.)
doi: 10.6023/cjoc201810002 |
|
|
(o) Zhu, F.; Wang, Y.; He, M.; Yan, Z.; Lin, S. Chin. J. Org. Chem. 2019, 39, 1175 (in Chinese).
doi: 10.6023/cjoc201810002 |
|
|
(朱福元, 王彦梅, 何明闯, 严兆华, 林森, 有机化学, 2019, 39, 1175.)
doi: 10.6023/cjoc201810002 |
|
| [16] |
(a) Hao, S.; Li, L.; Dong, D.; Wang, Z. Chin. J. Catal. 2017, 38, 1664.
doi: 10.6023/cjoc201901023 |
|
(b) Dong, D.; Hao, S.; Yang, D.; Li, L.; Wang, Z. Eur. J. Org. Chem. 2017, 6576.
doi: 10.6023/cjoc201901023 |
|
|
(c) Dong, D.; Zhang, H.; Wang, Z. RSC Adv. 2017, 7, 3780.
doi: 10.6023/cjoc201901023 |
|
|
(d) Zhang, H.; Dong, D.; Hao, S.; Wang, Z. RSC Adv. 2016, 6, 8465.
doi: 10.6023/cjoc201901023 |
|
|
(e) Dong, D. Q.; Chen, W. J.; Yang, Y.; Gao, X.; Wang, Z. L. ChemistrySelect 2019, 4, 2480.
doi: 10.6023/cjoc201901023 |
|
|
(f) Hao, S.; Li, L.; Dong, D.; Wang, Z.; Yu, X. Tetrahedron Lett. 2018, 59, 4073.
doi: 10.6023/cjoc201901023 |
|
|
(g) Li, G. H.; Dong, D. Q.; Yu, X. Y.; Wang, Z. L. New J. Chem. 2019, 43, 1667.
doi: 10.6023/cjoc201901023 |
|
|
(h) Li, G. H.; Dong, D. Q.; Yang, Y.; Yu, X. Y.; Wang, Z. L. Adv. Synth. Catal. 2019, 361, 832.
doi: 10.6023/cjoc201901023 |
|
|
(i) Yan, S.; Dong, D.-Q.; Xie, C.; Wang, W.; Wang, Z.-L. Chin. J. Org. Chem. 2019, 39, 2560 (in Chinese).
doi: 10.6023/cjoc201901023 |
|
|
(颜世强, 董道青, 解春文, 王文笙, 王祖利, 有机化学, 2019, 39, 2560.)
doi: 10.6023/cjoc201901023 |
|
|
(j) Li, G. H.; Dong, D. Q.; Deng, Q.; Yan, S. Q.; Wang, Z. L. Synthesis 2019, 51, 3313..
doi: 10.6023/cjoc201901023 |
|
|
(k) Xie, L. Y.; Peng, S.; Liu, F.; Chen, G. R.; Xia, W.; Yu, X.; Li, W. F.; Cao, Z.; He, W. M. Org. Chem. Front. 2018, 5, 2604.
doi: 10.6023/cjoc201901023 |
|
|
(l) Xie, L. Y.; Peng, S.; Jiang, L. L.; Peng, X.; Xia, W.; Yu, X.; Wang, X. X.; Cao, Z.; He, W. M. Org. Chem. Front. 2019, 2, 167.
doi: 10.6023/cjoc201901023 |
|
|
(m) Xu, X. M.; Chen, D. M.; Wang, Z. L. Chin. Chem. Lett. 2019,
doi: 10.6023/cjoc201901023 |
|
|
(n)Dong, D.Q.; Hao, S.Zhang, H.; Wang,Z.L.Chin.Chem.Lett.2017,28, 1597.
doi: 10.6023/cjoc201901023 |
|
| [17] |
Qiao L. Wei Y. Hao S. Chin. J. Org. Chem. 2018 38 509.
doi: 10.6023/cjoc201708004 |
|
乔 丽丽 魏 艳 郝 双红 有机化学 2018 38 509.
doi: 10.6023/cjoc201708004 |
|
| [18] |
Jin H. Geng Y. Yu Z. Tao K. Hou T. Pestic. Biochem. Physiol. 2009 93 133.
doi: 10.1016/j.pestbp.2009.01.002 |
| [1] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [2] | 霍海波, 李桂霞, 王世军, 韩春, 师宝君, 李健. 新型γ-咔啉衍生物的合成及其抑菌活性研究[J]. 有机化学, 2024, 44(1): 204-215. |
| [3] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [4] | 雷容超, 兰文捷, 李梦竹, 傅滨. 苯并磺内酰胺联吡唑化合物的简便合成[J]. 有机化学, 2023, 43(7): 2553-2560. |
| [5] | 许晓萍, 张翼飞, 莫小渝, 江俊. 铑催化3-重氮吲哚-2-亚胺与吡唑啉酮的C—H官能团化反应制备3-吡唑基吲哚[J]. 有机化学, 2023, 43(7): 2519-2527. |
| [6] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [7] | 时广辉, 杜云哲, 高媛媛, 贾慧劼, 洪海龙, 韩利民, 竺宁. 硫化物还原硝基在胺类合成中的应用[J]. 有机化学, 2023, 43(2): 491-502. |
| [8] | 周五, 彭敏, 梁庆祥, 吴爱斌, 舒文明, 余维初. 高选择和高灵敏检测溶液和气相中硫化氢的新型萘酰亚胺类开启型荧光探针[J]. 有机化学, 2023, 43(12): 4277-4283. |
| [9] | 赵瑜, 段玉荣, 史时辉, 白育斌, 黄亮珠, 杨晓军, 张琰图, 冯彬, 张建波, 张秋禹. 可见光促进高价碘(III)试剂参与反应的研究进展[J]. 有机化学, 2023, 43(12): 4106-4140. |
| [10] | 秦思凝. 芳香卤代物C—S偶联反应的研究进展[J]. 有机化学, 2023, 43(11): 3761-3783. |
| [11] | 吴宇恒, 颜岩, 寮渭巍. 双功能二氧化硫替代物在合成磺酰类化合物中的研究进展[J]. 有机化学, 2023, 43(11): 3713-3727. |
| [12] | 魏琬絜, 詹磊, 高雷, 黄国保, 马献力. 电化学合成C-磺酰基化合物的研究进展[J]. 有机化学, 2023, 43(1): 17-35. |
| [13] | 杨雅馨, 陈琳, 胡晓玲, 钟克利, 李世迪, 燕小梅, 张璟琳, 汤立军. 一种点亮型硫化氢荧光探针的合成及其在红酒和细胞中的应用[J]. 有机化学, 2023, 43(1): 308-312. |
| [14] | 汪蕾, 于淑晶, 杨娜, 王宝雷. 新型含二氢喹唑啉酮的咖啡因衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(1): 299-307. |
| [15] | 危斌, 周子龙, 秦景灏, 严泽宇, 郭嘉程, 雷澍, 谢叶香, 欧阳旋慧, 宋仁杰. 氧杂蒽与亚磺酸钠的电化学氧化C(sp3)—H磺酰化反应[J]. 有机化学, 2023, 43(1): 186-194. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||