有机化学 ›› 2022, Vol. 42 ›› Issue (1): 242-248.DOI: 10.6023/cjoc202106023 上一篇 下一篇
研究论文
王璐a, 邵莺a, 陈帆b, 钱鹏程b,*( ), 成江a,b,*(
), 成江a,b,*( )
)
收稿日期:2021-06-10
修回日期:2021-07-14
发布日期:2021-09-02
通讯作者:
钱鹏程, 成江
基金资助:
Lu Wanga, Ying Shaoa, Fan Chenb, Pengcheng Qianb( ), Jiang Chenga,b(
), Jiang Chenga,b( )
)
Received:2021-06-10
Revised:2021-07-14
Published:2021-09-02
Contact:
Pengcheng Qian, Jiang Cheng
Supported by:文章分享
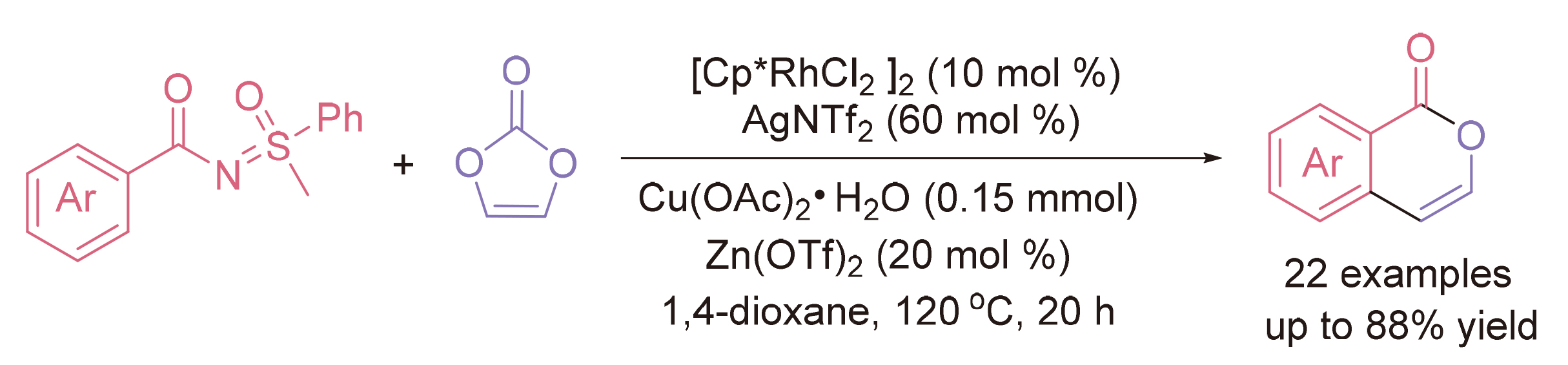
异香豆素是一种重要的药物分子, 目前已经开发了许多合成途径, 主要集中在苯甲酸或其他物质与炔烃的环化.亚磺酰基苯甲酰胺在螯合辅助的芳烃邻位C—H官能化中可以轻松地制备, 碳酸亚乙烯酯可以建立无氧化剂的催化体系, 使碳酸作为唯一的副产物. 在铑催化剂的存在下, 亚磺酰基苯甲酰胺和碳酸亚乙烯酯之间形成了环化, 通过导向基团辅助的邻位C—H偶联和分子内醇解, 以中等至良好的产率获得未取代的异香豆素.
王璐, 邵莺, 陈帆, 钱鹏程, 成江. 铑催化定位基促进芳环C—H键和碳酸亚乙烯酯的缩合得到异香豆素[J]. 有机化学, 2022, 42(1): 242-248.
Lu Wang, Ying Shao, Fan Chen, Pengcheng Qian, Jiang Cheng. Rhodium-Catalyzed Directing Group-Assisted Annulation of Arene C—H Bond with Vinylene Carbonate toward Isocoumarins[J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 242-248.
| Entry | Catalyst | Oxidant | Additive 1 | Additive 2 | Yieldb |
|---|---|---|---|---|---|
| 1 | Cp*Rh(OAc)2•H2O | AgSbF6 | Cu(OAc)2•H2O | — | <5% |
| 2 | [Cp*IrCl2]2 | AgSbF6 | Cu(OAc)2•H2O | — | <5% |
| 3 | [Cp*RhCl2]2 | AgSbF6 | Cu(OAc)2•H2O | — | 15% |
| 4 | [Cp*RhCl2]2 | AgSbF6 | — | — | NR |
| 5 | [Cp*RhCl2]2 | AgBF4 | Cu(OAc)2•H2O | — | 22% |
| 6 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | — | 56%, 49%c, 51%d |
| 7 | [Cp*RhCl2]2 | AgNTf2 | Cu(OTf)2 | — | 39% |
| 8 | [Cp*RhCl2]2 | AgNTf2 | CuI | — | 45% |
| 9 | [Cp*RhCl2]2 | AgNTf2 | Zn(OAc)2•H2O | — | <5% |
| 10 | [Cp*RhCl2]2 | AgNTf2 | Zn(OTf)2 | — | 58% |
| 11 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | HOAc | 49% |
| 12 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | Zn(OTf)2 | 78%, 71%e, 64%f |
| 13 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | ZnCl2 | 54%, 65%g, 20%h |
| 14 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | Zn(OTf)2 | 66%i |
| 15 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | Zn(OTf)2 | 59%j |
| 16 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | Zn(OTf)2 | 62%k |
| Entry | Catalyst | Oxidant | Additive 1 | Additive 2 | Yieldb |
|---|---|---|---|---|---|
| 1 | Cp*Rh(OAc)2•H2O | AgSbF6 | Cu(OAc)2•H2O | — | <5% |
| 2 | [Cp*IrCl2]2 | AgSbF6 | Cu(OAc)2•H2O | — | <5% |
| 3 | [Cp*RhCl2]2 | AgSbF6 | Cu(OAc)2•H2O | — | 15% |
| 4 | [Cp*RhCl2]2 | AgSbF6 | — | — | NR |
| 5 | [Cp*RhCl2]2 | AgBF4 | Cu(OAc)2•H2O | — | 22% |
| 6 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | — | 56%, 49%c, 51%d |
| 7 | [Cp*RhCl2]2 | AgNTf2 | Cu(OTf)2 | — | 39% |
| 8 | [Cp*RhCl2]2 | AgNTf2 | CuI | — | 45% |
| 9 | [Cp*RhCl2]2 | AgNTf2 | Zn(OAc)2•H2O | — | <5% |
| 10 | [Cp*RhCl2]2 | AgNTf2 | Zn(OTf)2 | — | 58% |
| 11 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | HOAc | 49% |
| 12 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | Zn(OTf)2 | 78%, 71%e, 64%f |
| 13 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | ZnCl2 | 54%, 65%g, 20%h |
| 14 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | Zn(OTf)2 | 66%i |
| 15 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | Zn(OTf)2 | 59%j |
| 16 | [Cp*RhCl2]2 | AgNTf2 | Cu(OAc)2•H2O | Zn(OTf)2 | 62%k |
| [1] |
(a) Saeed, A. Eur. J. Med. Chem. 2016, 116, 290.
doi: 10.1016/j.ejmech.2016.03.025 |
|
(b) Brasholz, M.; Soergel, S.; Azap, C.; Reissig, H.-U. Eur. J. Org. Chem. 2007, 3801.
|
|
|
(c) Pochet, L.; Frederick, R.; Masereel, B. Curr. Pharm. Des. 2004, 10, 3781.
doi: 10.2174/1381612043382684 |
|
|
(d) Wang, X.; Wedge, D. E.; Cutler, S. J. Nat. Prod. Commun. 2016, 11, 1595.
|
|
| [2] |
(a) Harunari, E.; Imada, C.; Igarashi, Y. J. Nat. Prod. 2019, 82, 1609.
doi: 10.1021/acs.jnatprod.9b00107 pmid: 31181919 |
|
(b) Das, P.; Babbar, P.; Malhotra, N.; Sharma, M.; Jachak, G. R.; Gonnade, R. G.; Shanmugam, D.; Harlos, K.; Yogavel, M.; Sharma, A.; Reddy, D. S. J. Med. Chem. 2018, 61, 5664.
doi: 10.1021/acs.jmedchem.8b00565 pmid: 31181919 |
|
| [3] |
(a) Hu, Z.; Wu, Z.; Su, Q.; Li, M.; Wu, S.; Meng, R.; Ding, W.; Li, C. Bioorg. Chem. 2020, 104, 104300.
doi: 10.1016/j.bioorg.2020.104300 |
|
(b) Zhou, J.; Zheng, L.; Hei, Z.; Li, W.; Wang, J.; Yu, B.; Fang, P. ACS Chem. Biol. 2020, 15, 1016.
doi: 10.1021/acschembio.0c00032 |
|
|
(c) Guo, D-D.; Zhang, W.-X.; Wang, Y.-Q. Chin. J. Org. Chem. 2019, 39, 2650. (in Chinese)
doi: 10.6023/cjoc201906001 |
|
|
(郭冬冬, 张武霞, 王永强, 有机化学, 2019, 39, 2650.)
doi: 10.6023/cjoc201906001 |
|
| [4] |
(a) Kumar, J. S.; Thirupataiah, B.; Medishetti, R.; Ray, A.; Bele, S. D.; Hossain, K. A.; Reddy, G. S.; Edwin, R. K.; Joseph, A.; Kumar, N.; Shenoy, G. G.; Rao, C. M.; Pal, M. Eur. J. Med. Chem. 2020, 201, 112335.
doi: 10.1016/j.ejmech.2020.112335 |
|
(b) Li, X.; Zhao, C.; Jing, S.; Sun, J.; Li, X.; Man, S.; Wang, Y.; Gao, W. Bioorg. Med. Chem. Lett. 2017, 27, 3595.
|
|
| [5] |
Hu, Z.-X.; Xue, Y.-B.; Bi, X.-B.; Zhang, J.-W.; Luo, Z.-W.; Li, X.-N.; Yao, G.-M.; Wang, J.-P.; Zhang, Y.-H. Mar. Drugs 2014, 12, 5563.
doi: 10.3390/md12115563 |
| [6] |
(a) Thirupataiah, B.; Reddy, G. S.; Ghule, S. S.; Kumar, J. S.; Mounika, G.; Hossain, K. A.; Mudgal, J.; Mathew, J. E.; Shenoy, G. G.; Parsa, K. V. L.; Pal, M. Bioorg. Chem. 2020, 97, 103691.
doi: S0045-2068(19)31615-3 pmid: 32143019 |
|
(b) Yang, Z.-N.; Su, B.-J.; Wang, Y.-Q.; Liao, H.-B.; Chen, Z.-F.; Liang, D. J. Nat. Prod. 2020, 83, 985.
doi: 10.1021/acs.jnatprod.9b00877 pmid: 32143019 |
|
|
(c) Gu, L.; Liu, C.; Fang, X.; Weng, Z.; Li, Z.; Tan, Y.; Tang, K. Chin. J. Synth. Chem. 2020, 28, 841. (in Chinese)
pmid: 32143019 |
|
|
(辜玲慧, 刘长英, 方新月, 翁正云, 李喆宇, 谭玉强, 唐克慧, 合成化学, 2020, 28, 841.)
pmid: 32143019 |
|
| [7] |
For reviews, please see: (a) Chen, G.; Yu, Y.; Huang, X. Synlett 2018, 29, 2087.
doi: 10.1055/s-0037-1610025 |
|
(b) Saikia, P.; Gogoi, S. Adv. Synth. Catal. 2018, 360, 2063.
doi: 10.1002/adsc.v360.11 |
|
|
(c) Saeed, A.; Haroon, M.; Muhammad, F.; Larik, F. A.; Hesham, E.-S.; Channar, P. A. J. Organomet. Chem. 2017, 834, 88.
doi: 10.1016/j.jorganchem.2017.02.016 |
|
|
(d) Saeed, A.; Larik, F. A. Chem. Heterocycl. Compd. 2016, 52, 450.
doi: 10.1007/s10593-016-1911-x |
|
|
(e) Ashraf, Z. Chem. Heterocycl. Compd. 2016, 52, 149.
doi: 10.1007/s10593-016-1849-z |
|
|
(f) Pal, S.; Chatare, V.; Pal, M. Curr. Org. Chem. 2011, 15, 782.
doi: 10.2174/138527211794518970 |
|
| [8] |
For selected recently examples, please see: (a) Liu, G.; Kuang, G.; Zhang, X.; Lu, N.; Fu, Y.; Peng, Y.; Zhou, Y. Org. Lett. 2019, 21, 3043.
doi: 10.1021/acs.orglett.9b00572 pmid: 28448155 |
|
(b) Jiang, G.; Li, J.; Zhu, C.; Wu, W.; Jiang, H. Org. Lett. 2017, 19, 4440.
doi: 10.1021/acs.orglett.7b01919 pmid: 28448155 |
|
|
(c) Mandal, R.; Sundararaju, B. Org. Lett. 2017, 19, 2544.
doi: 10.1021/acs.orglett.7b00801 pmid: 28448155 |
|
|
(d) Kudo, E.; Shibata, Y.; Yamazaki, M.; Masutomi, K.; Miyauchi, Y.; Fukui, M.; Sugiyama, H.; Uekusa, H.; Satoh, T.; Miura, M.; Tanaka, K. Chem.-Eur. J. 2016, 22, 14190.
doi: 10.1002/chem.201603499 pmid: 28448155 |
|
|
(e) Guo, X.-X. J. Org. Chem. 2013, 78, 166.
pmid: 28448155 |
|
| [9] |
With alcohol: (a) Luo, M.-J.; Hu, M.; Song, R.-J.; He, D.-L.; Li, J.-H. Chem. Commun. 2019, 55, 1124.
doi: 10.1039/C8CC08759H pmid: 31099571 |
|
With aldehyde: (b) Tao, L.-M.; Li, C.-H.; Chen, J.; Liu, H. J. Org. Chem. 2019, 84, 6807.
doi: 10.1021/acs.joc.9b00580 pmid: 31099571 |
|
|
With aldehyde: (c) Prakash, R.; Shekarrao, K.; Gogoi, S.; Boruah, R. C. Chem. Commun. 2015, 51, 9972.
doi: 10.1039/C5CC03311J pmid: 31099571 |
|
| [10] |
Mihara, G.; Ghosh, K.; Nishii, Y.; Miura, M. Org. Lett. 2020, 22, 5706.
doi: 10.1021/acs.orglett.0c02112 pmid: 32638595 |
| [11] |
For examples on the annulation of vinylene carbonate: (a) Li, X.; Huang, T.; Song, Y.; Qi, Y.; Li, L.; Li, Y.; Xiao, Q.; Zhang, Y. Org. Lett. 2020, 22, 5925.
doi: 10.1021/acs.orglett.0c02016 |
|
(b) Ghosh, K.; Nishii, Y.; Miura, M. Org. Lett. 2020, 22, 3547.
doi: 10.1021/acs.orglett.0c00975 |
|
|
(c) Ghosh, K.; Nishii, Y.; Miura, M. ACS Catal. 2019, 9, 11455.
doi: 10.1021/acscatal.9b04254 |
|
| [12] |
(a) Raghuvanshi, K.; Zell, D.; Ackermann, L. Org. Lett. 2017, 19, 1278.
doi: 10.1021/acs.orglett.6b03898 pmid: 22461080 |
|
(b) Shankar, M.; Ghosh, K.; Mukherjee, K.; Rit, R. K.; Sahoo, A. K. Org. Lett. 2018, 20, 5144.
doi: 10.1021/acs.orglett.8b02068 pmid: 22461080 |
|
|
(c) Rit, R. K.; Ramu Yadav, M.; Sahoo, A. K. Org. Lett. 2012, 14, 3724.
doi: 10.1021/ol301579q pmid: 22461080 |
|
|
(d) Ramu Yadav, M.; Rit, R. K.; Sahoo, A. K. Org. Lett. 2013, 15, 1638.
doi: 10.1021/ol400411v pmid: 22461080 |
|
|
(e) Ramu Yadav, M.; Rit, R. K.; Sahoo, A. K. Chem.-Eur. J. 2012, 18, 5541.
doi: 10.1002/chem.201200092 pmid: 22461080 |
|
| [13] |
(a) Yadav, M. R.; Rit, R. K.; Shankar, M.; Sahoo, A. K. J. Org. Chem. 2014, 79, 6123.
doi: 10.1021/jo5008465 pmid: 24905413 |
|
(b) Yadav, M. R.; Shankar, M.; Ramesh, E.; Ghosh, K.; Sahoo, A. K. Org. Lett. 2015, 17, 886.
pmid: 24905413 |
|
| [14] |
Santhi, J.; Baire, B. Chem. Asian J. 2019, 14, 3161.
doi: 10.1002/asia.v14.18 |
| [15] |
Toure, M.; Jaime-Figueroa, S.; Burslem, G. M.; Crews, C. M. Eur. J. Org. Chem. 2016, 4171.
|
| [16] |
Izumi, T.; Nishimoto, Y.; Kohei, K.; Kasahara, A. J. Heterocycl. Chem. 1990, 27, 1419.
doi: 10.1002/jhet.v27:5 |
| [17] |
Colonge, J.; Boisde, P. C. R. Hebd. Seances Acad. Sci. 1954, 239, 1047.
|
| [18] |
Kinder, M. A.; Kopf, J.; Margaretha, P. Tetrahedron 2000, 56, 6763.
doi: 10.1016/S0040-4020(00)00498-1 |
| [19] |
Mal, D.; Bandyopadhyay, M.; Ghorai, S. K.; Datta, K. Tetrahedron Lett. 2000, 41, 3677.
doi: 10.1016/S0040-4039(00)00441-X |
| [1] | 文思, 丁宇浩, 田青于, 葛进, 程国林. 铑(III)催化苯甲亚胺酸乙酯和CF3-亚胺氧锍叶立德C—H 活化/环化反应合成CF3-1H-苯并[de][1,8]萘吡啶[J]. 有机化学, 2024, 44(1): 291-300. |
| [2] | 高晓阳, 翟锐锐, 陈训, 王烁今. 碳酸亚乙烯酯参与C—H键活化反应的研究进展[J]. 有机化学, 2023, 43(9): 3119-3134. |
| [3] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [4] | 张彦波, 孙萌. 铑催化碳酸亚乙烯酯与吲哚啉C(7)位C—H甲酰甲基化反应[J]. 有机化学, 2023, 43(8): 2905-2912. |
| [5] | 汤振, 皮超, 吴养洁, 崔秀灵. 铑催化2-芳基-2H-吲唑与硫叶立德的酰甲基化/串联环化反应高效构建6-芳基吲唑并[2,3-a]喹啉类衍生物[J]. 有机化学, 2023, 43(3): 1187-1196. |
| [6] | 刘晓洁, 徐必平, 苏伟平. 铑催化羧酸原位生成酰氟的脱羰Suzuki-Miyaura偶联[J]. 有机化学, 2022, 42(7): 2184-2191. |
| [7] | 韩高旭, 许红涛, 侯卫. 铑(III)催化的C(sp3)—H官能团化[J]. 有机化学, 2022, 42(2): 391-423. |
| [8] | 王家状, 滕丽果, 熊绍棋, 肖铁波, 江玉波. Rh催化N-磺酰腙的偕-二氟烯丙基化反应[J]. 有机化学, 2022, 42(11): 3658-3667. |
| [9] | 李芳洁, 卢斌, 刘阳, 王晓明. 双铑(II)/Xantphos催化的C—H官能团化/烯丙基烷基化串联: 从N-芳基-α-重氮-β-酮酯简便制备3-酰基-3-烯丙基氧化吲哚衍生物[J]. 有机化学, 2022, 42(10): 3390-3397. |
| [10] | 徐曼, 夏远志. 铑(III)催化N-苯氧基乙酰胺与亚甲基氧杂环丁酮氧化还原中性的碳氢活化/环化反应的机理研究[J]. 有机化学, 2021, 41(8): 3272-3278. |
| [11] | 戴雨倩, 李兴伟, 刘丙贤. 三价铑催化通过环己二酮高效构建异香豆素类化合物[J]. 有机化学, 2021, 41(11): 4476-4483. |
| [12] | 宗玲博, 陈建宾, 任新意, 张国营, 贾肖飞. 有机聚合物负载铑催化剂在氢甲酰化反应中的应用研究进展[J]. 有机化学, 2020, 40(8): 2308-2321. |
| [13] | 赵森, 李淳朴, 许斌, 柳红. 铑催化碳氢二氟烯丙基化/N-碘代丁二酰亚胺介导的环化反应构建含氟3,4-二氢嘧啶并[1,6-a]吲哚-1(2H)-酮衍生物[J]. 有机化学, 2020, 40(6): 1549-1562. |
| [14] | 刘金宝, 李鹏, 姚子健. 含半夹心铱/铑/钌结构基元的离散型金属环状化合物的研究进展[J]. 有机化学, 2020, 40(2): 364-375. |
| [15] | 陈亮, 胡良建, 杜宇, 苏伟平, 康强. 中心手性金属铑配合物催化的不对称光诱导Giese自由基加成反应[J]. 有机化学, 2020, 40(11): 3944-3952. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||