有机化学 ›› 2023, Vol. 43 ›› Issue (1): 171-177.DOI: 10.6023/cjoc202208014 上一篇 下一篇
研究论文
收稿日期:2022-08-13
修回日期:2022-09-02
发布日期:2022-10-10
通讯作者:
徐晶
基金资助:
Jingping Hua,b, Wenqing Chenb, Yuyang Jiangb, Jing Xub,c( )
)
Received:2022-08-13
Revised:2022-09-02
Published:2022-10-10
Contact:
Jing Xu
Supported by:文章分享
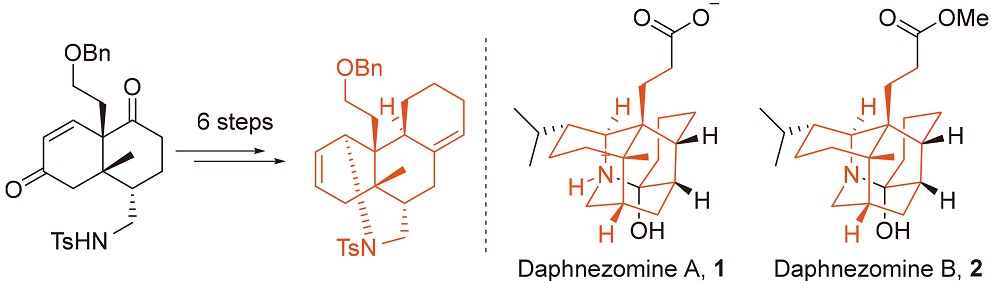
在虎皮楠生物碱家族中, daphnezomine A型生物碱仅包含三位成员: daphnezomine A (1), daphnezomine B (2)以及dapholdhamine B. 这些生物碱含有独特的氮杂金刚烷骨架, 9个连续的手性立体中心, 因此呈现出巨大的合成挑战性. 报道了分子1和2的四环核心骨架的合成. 关键步骤包括一个黄氏酰胺活化增环反应和一个Hutchins-Kabalka还原重排反应.
胡晶平, 陈文清, 蒋宇旸, 徐晶. Daphnezomines A和B的四环核心骨架合成[J]. 有机化学, 2023, 43(1): 171-177.
Jingping Hu, Wenqing Chen, Yuyang Jiang, Jing Xu. Synthesis of Tetracyclic Core Structure of Daphnezomines A and B[J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 171-177.
| [1] |
(a) Li, Z.-Y.; Guo, Y.-W. Chin. J. Org. Chem. 2007, 27, 565. (in Chinese)
doi: 10.1002/cjoc.200990092 |
|
(李震宇, 郭跃伟, 有机化学, 2007, 27, 565.)
|
|
|
(b) Liang, X.; Yang, X.-Z.; Chen, L.; Jiang, S.; Chen, Y.-D.; Deng, Q.-Y.; Chen, X.-G.; Yuan, J.-Q. Med. Chem. Res. 2020, 30, 1.
doi: 10.1007/s00044-020-02646-w |
|
|
(c) Wu, H.; Zhang, X.; Ding, L.; Chen, S.; Yang, J.; Xu, X. Planta Med. 2013, 79, 1589.
doi: 10.1055/s-0033-1351024 |
|
|
(d) Xu, J. B.; Zhang, H.; Gan, L. S.; Han, Y. S.; Wainberg, M. A.; Yue, J. M. J. Am. Chem. Soc. 2014, 136, 7631.
doi: 10.1021/ja503995b |
|
| [2] |
(a) Kobayashi, J.; Kubota, T. Nat. Prod. Rep. 2009, 26, 936.
doi: 10.1039/b813006j pmid: 28205435 |
|
(b) Chattopadhyay, A. K.; Hanessian, S. Chem. Rev. 2017, 117, 4104.
doi: 10.1021/acs.chemrev.6b00412 pmid: 28205435 |
|
|
(c) Kang, B.; Jakubec, P.; Dixon, D. J. Nat. Prod. Rep. 2014, 31, 550.
doi: 10.1039/C3NP70115H pmid: 28205435 |
|
|
(d) Guo, L.-D.; Chen, Y.; Xu, J. Acc. Chem. Res. 2020, 53, 2726.
doi: 10.1021/acs.accounts.0c00532 pmid: 28205435 |
|
|
(e) Zhong, J.; Wang, H.; Zhang, Q.; Gao, S. Alkaloids: Chem. Biol. 2021, 85, 113.
pmid: 28205435 |
|
| [3] |
(a) Piettre, S.; Heathcock, C. H. Science 1990, 248, 1532.
pmid: 17818314 |
|
(b) Heathcock, C. H.; Davidsen, S. K.; Mills, S.; Sanner, M. A. J. Am. Chem. Soc. 1986, 108, 5650.
doi: 10.1021/ja00278a061 pmid: 17818314 |
|
|
(c) Ruggeri, R. B.; Heathcock, C. H. J. Org. Chem. 1990, 55, 3714.
doi: 10.1021/jo00299a006 pmid: 17818314 |
|
|
(d) Heathcock, C. H.; Kath, J. C.; Ruggeri, R. B. J. Org. Chem. 1995, 60, 1120.
doi: 10.1021/jo00110a013 pmid: 17818314 |
|
|
(e) Ruggeri, R. B.; Hansen, M. M.; Heathcock, C. H. J. Am. Chem. Soc. 1988, 110, 8734.
doi: 10.1021/ja00234a046 pmid: 17818314 |
|
|
(f) Stafford, J. A.; Heathcock, C. H. J. Org. Chem. 1990, 55, 5433.
doi: 10.1021/jo00307a006 pmid: 17818314 |
|
|
(g) Heathcock, C. H.; Stafford, J. A.; Clark, D. L. J. Org. Chem. 1992, 57, 2575.
doi: 10.1021/jo00035a011 pmid: 17818314 |
|
|
(h) Ruggeri, R. B.; McClure, K. F.; Heathcock, C. H. J. Am. Chem. Soc. 1989, 111, 1530.
doi: 10.1021/ja00186a075 pmid: 17818314 |
|
| [4] |
Weiss, M. E.; Carreira, E. M. Angew. Chem., Int. Ed. 2011, 50, 11501.
doi: 10.1002/anie.201104681 |
| [5] |
(a) Lu, Z. Y.; Li, Y.; Deng, J.; Li, A. Nat. Chem. 2013, 5, 679.
doi: 10.1038/nchem.1694 |
|
(b) Li, J.; Zhang, W. H.; Zhang, F.; Chen, Y.; Li, A. J. Am. Chem. Soc. 2017, 139, 14893.
doi: 10.1021/jacs.7b09186 |
|
|
(c) Chen, Y.; Zhang, W. H.; Ren, L.; Li, J.; Li, A. Angew. Chem., Int. Ed. 2018, 57, 952.
doi: 10.1002/anie.201711482 |
|
|
(d) Zhang, W. H.; Ding, M.; Li, J.; Guo, Z. C.; Lu, M.; Chen, Y.; Liu, L. C.; Shen, Y. H.; Li, A. J. Am. Chem. Soc. 2018, 140, 4227.
doi: 10.1021/jacs.8b01681 |
|
| [6] |
(a) Shvartsbart, A.; Smith, A. B., III J. Am. Chem. Soc. 2014, 136, 870.
doi: 10.1021/ja411539w pmid: 25756504 |
|
(b) Shvartsbart, A.; Smith, A. B., III J. Am. Chem. Soc. 2015, 137, 3510.
doi: 10.1021/ja503899t pmid: 25756504 |
|
| [7] |
Yamada, R.; Adachi, Y.; Yokoshima, S.; Fukuyama, T. Angew. Chem.,Int. Ed. 2016, 55, 6067.
doi: 10.1002/anie.201601958 |
| [8] |
Shi, H.; Michaelides, I. N.; Darses, B.; Jakubec, P.; Nguyen, Q. N. N.; Paton, R. S.; Dixon, D. J. J. Am. Chem. Soc. 2017, 139, 17755.
doi: 10.1021/jacs.7b10956 |
| [9] |
Chen, X.; Zhang, H.-J.; Yang, X.; Lv, H.; Shao, X.; Tao, C.; Wang, H.; Cheng, B.; Li, Y.; Guo, J.; Zhang, J.; Zhai, H. Angew. Chem.,Int. Ed. 2018, 57, 947.
doi: 10.1002/anie.201709762 |
| [10] |
(a) Xu, B.; Wang, B.; Xun, W.; Qiu, F. G. Angew. Chem.,Int. Ed. 2019, 58, 5754.
doi: 10.1002/anie.201902268 |
|
(b) Wang, B.; Xu, B.; Xun, W.; Guo, Y.; Zhang, J.; Qiu, F. G. Angew. Chem., Int. Ed. 2021, 60, 9439.
doi: 10.1002/anie.202016212 |
|
| [11] |
Zhong, J.; Chen, K.; Qiu, Y.; He, H.; Gao, S. Org. Lett. 2019, 21, 3741.
doi: 10.1021/acs.orglett.9b01184 |
| [12] |
(a) Hugelshofer, C. L.; Palani, V.; Sarpong, R. J. Am. Chem. Soc. 2019, 141, 8431.
doi: 10.1021/jacs.9b03576 pmid: 31533427 |
|
(b) Hugelshofer, C. L.; Palani, V.; Sarpong, R. J. Org. Chem. 2019, 84, 14069.
doi: 10.1021/acs.joc.9b02223 pmid: 31533427 |
|
| [13] |
Xu, G.; Wu, J.; Li, L.; Lu, Y.; Li, C. J. Am. Chem. Soc. 2020, 142, 15240.
doi: 10.1021/jacs.0c06717 |
| [14] |
Cao, M.-Y.; Ma, B.-J.; Gu, Q.-X.; Fu, B.; Lu, H.-H. J. Am. Chem. Soc. 2022, 144, 5750.
doi: 10.1021/jacs.2c01674 |
| [15] |
Chattopadhyay, A. K.; Ly, V. L.; Jakkepally, S.; Berger, G.; Hanessian, S. Angew. Chem.,Int. Ed. 2016, 55, 2577.
doi: 10.1002/anie.201510861 |
| [16] |
(a) Chen, Y.; Hu, J.; Guo, L.-D.; Zhong, W.; Ning, C.; Xu, J. Angew. Chem., Int. Ed. 2019, 58, 7390.
doi: 10.1002/anie.201902908 |
|
(b) Guo, L.-D.; Hou, J.; Tu, W.; Zhang, Y.; Zhang, Y.; Chen, L.; Xu, J. J. Am. Chem. Soc. 2019, 141, 11713.
doi: 10.1021/jacs.9b05641 |
|
|
(c) Guo, L.-D.; Hu, J.; Zhang, Y.; Tu, W.; Zhang, Y.; Pu, F.; Xu, J. J. Am. Chem. Soc. 2019, 141, 13043.
doi: 10.1021/jacs.9b07558 |
|
|
(d) Guo, L.-D.; Zhang, Y.; Hu, J.; Ning, C.; Fu, H.; Chen, Y.; Xu, J. Nat. Commun. 2020, 11, 3538.
doi: 10.1038/s41467-020-17350-x |
|
|
For our very recent total syntheses of other Daphniphyllum alkaloids, see:
|
|
|
(e) Zhang, Y.; Chen, Y.; Song, M.; Tan, B.; Jiang, Y.; Yan, C.; Jiang, Y.; Hu, X.; Zhang, C.; Chen, W.; Xu, J. J. Am. Chem. Soc. 2022, 144, 16042.
doi: 10.1021/jacs.2c05957 |
|
|
(f) Hu, J.; Guo, L.-D.; Chen, W.; Jiang, Y.; Pu, F.; Ning, C.; Xu, J. Org. Lett. 2022, 24, 7416.
doi: 10.1021/acs.orglett.2c02988 |
|
| [17] |
Morita, H.; Yoshida, N.; Kobayashi, J. J. Org. Chem. 1999, 64, 7208.
doi: 10.1021/jo990882o |
| [18] |
Zhang, Y.; Di, Y.-T.; Mu, S.-Z.; Li, C.-S.; Zhang, Q.; Tan, C.-J.; Zhang, Z.; Fang, X.; Hao, X.-J. J. Nat. Prod. 2009, 72, 1325.
doi: 10.1021/np900112d pmid: 19441852 |
| [19] |
(a) Wu, D.-P.; He, Q.; Chen, D.-H.; Ye, J.-L.; Huang, P.-Q. Chin. J. Chem. 2019, 37, 315.
doi: 10.1002/cjoc.201900035 |
|
(b) Huang, P.-Q.; Huang, Y.-H. Chin. J. Chem. 2017, 35, 613.
doi: 10.1002/cjoc.201600700 |
|
|
(c) Huang, P. -, Q.; Huang, Y.-H.; Wang, S.-R. Org. Chem. Front. 2017, 4, 431.
doi: 10.1039/C6QO00720A |
|
|
(d) Huang, P.-Q.; Huang, Y.-H.; Geng, H.; Ye, J.-L. Sci. Rep. 2016, 6, 28801.
doi: 10.1038/srep28801 |
|
| [20] |
(a) Hutchins, R. O.; Kacher, M.; Rua, L. J. Org. Chem. 1975, 40, 923.
doi: 10.1021/jo00895a024 |
|
(b) Kabalka, G. W.; Yang, D. T. C.; Baker, J. D. J. Org. Chem. 1976, 41, 574.
doi: 10.1021/jo00865a043 |
|
| [21] |
Crystallographic data for compound C9-epi-4 (CCDC 2170411) has been deposited at the Cambridge Crystallographic Data Centre.
|
| [22] |
Yang, J.; Xiong, Z.; Chen, Y.; Li, Y. Synth. Commun. 1997, 27, 2985.
doi: 10.1080/00397919708005005 |
| [23] |
Wang, Z. Meinwald Rearrangement. In Comprehensive Organic Name Reactions and Reagents, Wiley Online Library, Hoboken, NJ, USA, 2010, pp. 1880-1882.
|
| [24] |
Crystallographic data for compound 14 (CCDC 2170422) has been deposited at the Cambridge Crystallographic Data Centre.
|
| [25] |
For our recent synthesis and studies of other caged natural products, see:
pmid: 29364591 |
|
(a) Xie, S. Ning, C.; Yu, Q.; Hou, J.; Xu, J. Chin. J. Chem. 2021, 39, 137.
doi: 10.1002/cjoc.202000460 pmid: 29364591 |
|
|
(b) Xie, S.; Chen, G.; Yan, H.; Hou, J.; He, Y.; Zhao, T.; Xu, J. J. Am. Chem. Soc. 2019, 141, 3435.
doi: 10.1021/jacs.9b00391 pmid: 29364591 |
|
|
(c) Zhang, Y.; Guo, L.-D.; Xu, J. Chin. J. Org. Chem. 2019, 39, 1079. (in Chinese)
pmid: 29364591 |
|
|
(张焱, 郭联东, 徐晶, 有机化学, 2019, 39, 079.)
pmid: 29364591 |
|
|
(d) Zhao, N.; Yin, S.; Xie, S.; Yan, H.; Ren, P.; Chen, G.; Chen, F.; Xu, J. Angew. Chem. Int. Ed. 2018, 57, 3386.
doi: 10.1002/anie.201800167 pmid: 29364591 |
| [1] | 蔡荣斌, 李冰, 周琪, 朱隆懿, 罗军. 4,8,9,10-四官能化的2-氮杂金刚烷及其2-氮杂原金刚烷骨架异构体的合成[J]. 有机化学, 2023, 43(6): 2217-2225. |
| [2] | 刘兴周, 于明加, 梁建华. 原小檗碱骨架的合成及其抗炎活性研究进展[J]. 有机化学, 2023, 43(4): 1325-1340. |
| [3] | 孔祥凯, 张逸鹏, 党菱婧, 陈文, 张洪彬. 吲哚生物碱Vindoline与Vindorosine的合成研究进展[J]. 有机化学, 2022, 42(9): 2728-2744. |
| [4] | 高冉, 田伟生. 苦楝甾醇及2α,3α,20R-三羟基孕甾-16β-甲基丙烯酸酯的合成[J]. 有机化学, 2022, 42(8): 2521-2526. |
| [5] | 徐萌萌, 蔡泉. 2-吡喃酮的催化不对称Diels-Alder反应研究进展[J]. 有机化学, 2022, 42(3): 698-713. |
| [6] | 赵军, 肖检, 王雅雯, 彭羽. 基于氧化[3+2]环加成反应合成二氢苯并呋喃类天然产物[J]. 有机化学, 2021, 41(8): 2933-2945. |
| [7] | 李敏欣, 邹秋萍, 杜文绒, 高金春, 李艳平, 毛泽伟. Dorsmerunin A的全合成及其抗炎活性研究[J]. 有机化学, 2021, 41(8): 3292-3296. |
| [8] | 肖检, 彭羽, 李卫东. 倍半萜生物碱Dendrobine的全合成研究进展[J]. 有机化学, 2021, 41(7): 2636-2649. |
| [9] | 严锡飞, 郑剑峰, 李卫东. 天然药物小檗碱的化学合成研究进展[J]. 有机化学, 2021, 41(6): 2217-2227. |
| [10] | 杨刚, 冯翔宇, 韩丛丛, 陈洋, 何述钟. Wallichanol类天然产物ABC环系合成研究[J]. 有机化学, 2021, 41(2): 726-730. |
| [11] | 马丁, 敖军礼, 胡乃峰, 梁广鑫. 线虫孵化信息素Glycinoeclepin A的生物活性及合成研究进展[J]. 有机化学, 2021, 41(2): 553-566. |
| [12] | 郭璐, 汤平平. 五味子三萜天然产物的合成进展[J]. 有机化学, 2021, 41(10): 3816-3825. |
| [13] | 韩吉来, 唐美麟, 孙逊. 白藜芦醇二聚体Quadrangularin A和Pallidol合成方法学研究[J]. 有机化学, 2020, 40(6): 1571-1577. |
| [14] | 董玮, 王欣, 葛泽梅, 和芳, 李润涛. 汉黄芩素的方便合成[J]. 有机化学, 2020, 40(6): 1725-1730. |
| [15] | 谢涛, 何海兵, 高栓虎. 多环型Xanthone类天然产物的合成研究进展[J]. 有机化学, 2020, 40(3): 551-562. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||