有机化学 ›› 2023, Vol. 43 ›› Issue (3): 1012-1022.DOI: 10.6023/cjoc202211048 上一篇 下一篇
所属专题: 中国女科学家专辑
综述与进展
侯虹宇a,b, 程元元a,b, 陈彬a,b, 佟振合a,b, 吴骊珠a,b,*( )
)
收稿日期:2022-11-30
修回日期:2023-01-13
发布日期:2023-01-18
通讯作者:
吴骊珠
基金资助:
Hongyu Houa,b, Yuanyuan Chenga,b, Bin Chena,b, Chenho Tunga,b, Lizhu Wua,b( )
)
Received:2022-11-30
Revised:2023-01-13
Published:2023-01-18
Contact:
Lizhu Wu
Supported by:文章分享

烯烃酰化反应被广泛用于制备结构丰富且高附加值的酮、醛、羧酸及其衍生物. 多组分串联N-杂环卡宾催化和过渡金属催化的烯烃α-酰化反应具有高效、高选择性的优点, 然而受限的反应模式和底物范围限制了反应的发展. 光催化烯烃α-酰化反应突破了这一限制, 具有更丰富、更广泛的底物适用范围. 以研究策略为线索, 对这一快速的研究进展进行了总结和展望.
侯虹宇, 程元元, 陈彬, 佟振合, 吴骊珠. 光催化烯烃α-酰化反应[J]. 有机化学, 2023, 43(3): 1012-1022.
Hongyu Hou, Yuanyuan Cheng, Bin Chen, Chenho Tung, Lizhu Wu. α-Acylation of Olefins via Photocatalysis[J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1012-1022.
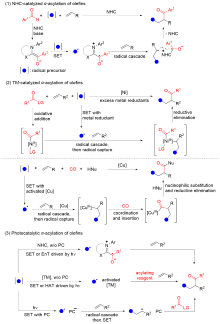
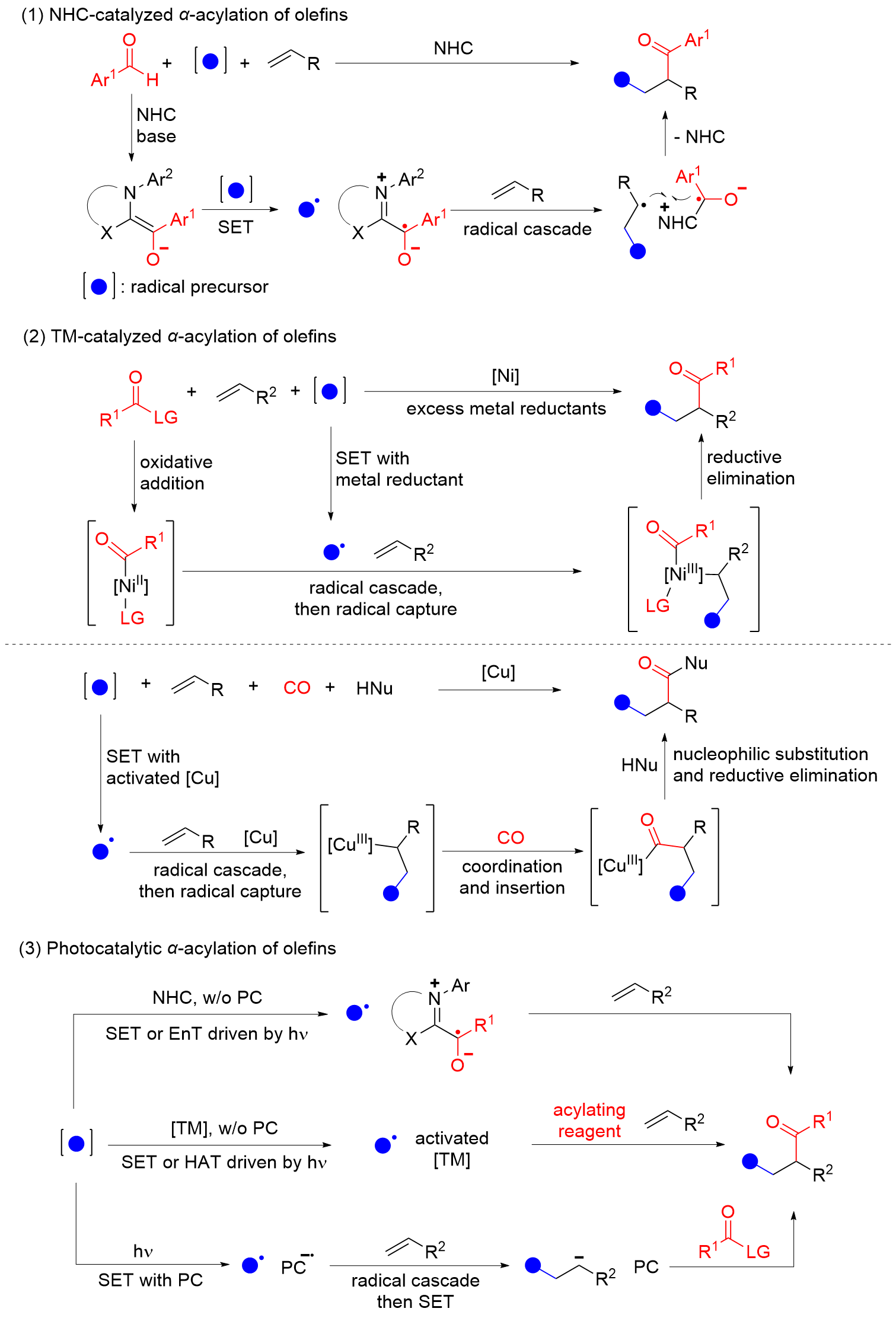
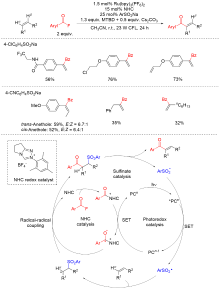

| [1] |
He, X. K.; Cai, B. G.; Yang, Q. Q.; Wang, L.; Xuan, J. Chem.-Asian J. 2019, 14, 3269.
doi: 10.1002/asia.v14.19 |
| [2] |
Biju, A. T.; Kuhl, N.; Glorius, F. Acc. Chem. Res. 2011, 44, 1182.
doi: 10.1021/ar2000716 |
| [3] |
Chatgilialoglu, C.; Crich, D.; Komatsu, M.; Ryu, I. Chem. Rev. 1999, 99, 1991.
doi: 10.1021/cr9601425 pmid: 11849018 |
| [4] |
Willis, M. C. Chem. Rev. 2010, 110, 725.
doi: 10.1021/cr900096x |
| [5] |
Li, J.-L.; Liu, Y.-Q.; Zou, W.-L.; Zeng, R.; Zhang, X.; Liu, Y.; Han, B.; He, Y.; Leng, H.-J.; Li, Q.-Z. Angew. Chem., Int. Ed. 2020, 59, 1863.
doi: 10.1002/anie.v59.5 |
| [6] |
Ishii, T.; Ota, K.; Nagao, K.; Ohmiya, H. J. Am. Chem. Soc. 2019, 141, 14073.
doi: 10.1021/jacs.9b07194 |
| [7] |
Matsuki, Y.; Ohnishi, N.; Kakeno, Y.; Takemoto, S.; Ishii, T.; Nagao, K.; Ohmiya, H. Nat. Commun. 2021, 12, 3848.
doi: 10.1038/s41467-021-24144-2 pmid: 34158509 |
| [8] |
Yang, H. B.; Wang, Z. H.; Li, J. M.; Wu, C. Chem. Commun. 2020, 56, 3801.
doi: 10.1039/D0CC00293C |
| [9] |
Zhang, B.; Peng, Q.; Guo, D.; Wang, J. Org. Lett. 2020, 22, 443.
doi: 10.1021/acs.orglett.9b04203 pmid: 31904244 |
| [10] |
Gao, Y.; Quan, Y.; Li, Z.; Gao, L.; Zhang, Z.; Zou, X.; Yan, R.; Qu, Y.; Guo, K. Org. Lett. 2021, 23, 183.
doi: 10.1021/acs.orglett.0c03907 pmid: 33336577 |
| [11] |
Li, Q. Z.; Liu, Y. Q.; Kou, X. X.; Zou, W. L.; Qi, T.; Xiang, P.; Xing, J. D.; Zhang, X.; Li, J. L. Angew. Chem., Int. Ed. 2022, 10.1002/anie.202207824.
|
| [12] |
Wang, L.; Wang, C. Org. Lett. 2020, 22, 8829.
doi: 10.1021/acs.orglett.0c03210 pmid: 33118826 |
| [13] |
Zhao, X.; Tu, H.-Y.; Guo, L.; Zhu, S.; Qing, F.-L.; Chu, L. Nat. Commun. 2018, 9, 3488.
doi: 10.1038/s41467-018-05951-6 |
| [14] |
Wu, F. P.; Yuan, Y.; Wu, X. F. Angew. Chem., Int. Ed. 2021, 60, 25787.
|
| [15] |
Christmann, M. Angew. Chem., Int. Ed. 2005, 44, 2632.
doi: 10.1002/anie.v44:18 |
| [16] |
Chakrabortty, S.; Almasalma, A. A.; de Vries, J. G. Catal. Sci. Technol. 2021, 11, 5388.
doi: 10.1039/D1CY00737H |
| [17] |
Ghosh, A.; Johnson, K. F.; Vickerman, K. L.; Walker, J. A.; Stanley, L. M. Org. Chem. Front. 2016, 3, 639.
doi: 10.1039/C6QO00023A |
| [18] |
Li, J.; Shi, Y. Chem. Soc. Rev. 2022, 51, 6757.
doi: 10.1039/D2CS00150K |
| [19] |
Meng, Q.-Y.; Dçben, N.; Studer, A. Angew. Chem., Int. Ed. 2020, 59, 19956.
doi: 10.1002/anie.v59.45 |
| [20] |
Liu, K.; Studer, A. J. Am. Chem. Soc. 2021, 143, 4903.
doi: 10.1021/jacs.1c01022 |
| [21] |
Liu, M.-S.; Min, L.; Chen, B.-H.; Shu, W. ACS Catal. 2021, 11, 9715.
doi: 10.1021/acscatal.1c02890 |
| [22] |
Du, H. W.; Liu, M. S.; Shu, W. Org. Lett. 2022, 24, 5519.
doi: 10.1021/acs.orglett.2c01915 |
| [23] |
Sato, Y.; Goto, Y.; Nakamura, K.; Miyamoto, Y.; Sumida, Y.; Ohmiya, H. ACS Catal. 2021, 11, 12886.
doi: 10.1021/acscatal.1c04153 |
| [24] |
Ren, S. C.; Yang, X.; Mondal, B.; Mou, C.; Tian, W.; Jin, Z.; Chi, Y. R. Nat. Commun. 2022, 13, 2846.
doi: 10.1038/s41467-022-30583-2 |
| [25] |
Jin, S.; Sui, X.; Haug, G. C.; Nguyen, V. D.; Dang, H. T.; Arman, H. D.; Larionov, O. V. ACS Catal. 2021, 12, 285.
doi: 10.1021/acscatal.1c04594 |
| [26] |
Wang, L.; Sun, J.; Xia, J.; Li, M.; Zhang, L.; Ma, R.; Zheng, G.; Zhang, Q. Sci. China: Chem. 2022, 65, 1938.
doi: 10.1007/s11426-022-1328-5 |
| [27] |
Zhao, X.; Li, B.; Xia, W. Org. Lett. 2020, 22, 1056.
doi: 10.1021/acs.orglett.9b04595 |
| [28] |
Liu, J.; Lu, L.-Q.; Luo, Y.; Zhao, W.; Sun, P.-C.; Jin, W.; Qi, X.; Cheng, Y.; Xiao, W.-J. ACS Catal. 2022, 12, 1879.
doi: 10.1021/acscatal.1c05672 |
| [29] |
Wang, D.; Ackermann, L. Chem. Sci. 2022, 13, 7256.
doi: 10.1039/D2SC02277J |
| [30] |
Luridiana, A.; Mazzarella, D.; Capaldo, L.; Rincón, J. A.; García-Losada, P.; Mateos, C.; Frederick, M. O.; Nuño, M.; Jan Buma, W.; Noël, T. ACS Catal. 2022, 12, 11216.
doi: 10.1021/acscatal.2c03805 pmid: 36158902 |
| [31] |
Zhang, Y.; Yuan, Y.; Geng, H.-Q.; Xu, J.-X.; Wu, X.-F. J. Catal. 2022, 413, 214.
doi: 10.1016/j.jcat.2022.06.033 |
| [32] |
Cheng, Y.-Y.; Yu, J.-X.; Lei, T.; Hou, H.-Y.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Angew. Chem., Int. Ed. 2021, 60, 26822.
doi: 10.1002/anie.v60.51 |
| [33] |
Li, S.; Shu, H.; Wang, S.; Yang, W.; Tang, F.; Li, X. X.; Fan, S.; Feng, Y. S. Org. Lett. 2022, 24, 5710.
doi: 10.1021/acs.orglett.2c02108 |
| [34] |
Cheng, Y.-Y.; Hou, H.-Y.; Liu, Y.; Yu, J.-X.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Angew. Chem., Int. Ed. 2022, 61, e202208831.
|
| [35] |
Fan, P.; Lan, Y.; Zhang, C.; Wang, C. J. Am. Chem. Soc. 2020, 142, 2180.
doi: 10.1021/jacs.9b12554 |
| [36] |
Liu, D.; Yang, K.; Fang, D.; Li, S. J.; Lan, Y.; Chen, Y. Angew. Chem., Int. Ed. 2022, 62, e202213686.
|
| [37] |
Chen, J. Q.; Tu, X.; Tang, Q.; Li, K.; Xu, L.; Wang, S.; Ji, M.; Li, Z.; Wu, J. Nat. Commun. 2021, 12, 5328.
doi: 10.1038/s41467-021-25628-x |
| [1] | 张剑, 梁万洁, 杨艺, 闫法超, 刘会. 联烯胺化合物的区域选择性双官能团化[J]. 有机化学, 2024, 44(2): 335-348. |
| [2] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [3] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [4] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [5] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [6] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [7] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [8] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [9] | 杨晓娜, 郭宏宇, 周荣. 可见光促进有机硅化合物参与的化学转化[J]. 有机化学, 2023, 43(8): 2720-2742. |
| [10] | 普佳霞, 贾小英, 韩丽荣, 李清寒. 可见光诱导C—N键断裂构建C—C键的研究进展[J]. 有机化学, 2023, 43(8): 2591-2613. |
| [11] | 王灵娜, 刘晓庆, 林钢, 金泓颖, 焦民均, 刘雪粉, 罗书平. 光促进双(4-二苯甲酮)苯醚催化C(sp3)—H键活化构建C—S键[J]. 有机化学, 2023, 43(8): 2848-2854. |
| [12] | 赵瑜, 张凯, 白育斌, 张琰图, 史时辉. 无金属条件下可见光催化与溴盐协同促进烯烃的氢硅化反应研究[J]. 有机化学, 2023, 43(8): 2837-2847. |
| [13] | 刘颖杰, 石岗庆, 仇格, 张鑫, 宋冬雪, 陈宁, 于淼, 许颖. 光/电催化醚α-位官能团化研究进展[J]. 有机化学, 2023, 43(8): 2664-2681. |
| [14] | 刘亚鑫, 张渔, 罗书平. 热延迟荧光(TADF)光敏剂的设计合成及其光催化脱卤反应性能研究[J]. 有机化学, 2023, 43(7): 2476-2483. |
| [15] | 陈宁, 张成栋, 李鹏, 仇格, 刘颖杰, 张天雷. 光/电化学驱动螺环化合物的合成研究进展[J]. 有机化学, 2023, 43(7): 2293-2303. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||