有机化学 ›› 2023, Vol. 43 ›› Issue (3): 1124-1135.DOI: 10.6023/cjoc202211038 上一篇 下一篇
所属专题: 中国女科学家专辑
研究论文
收稿日期:2022-11-29
修回日期:2023-01-04
发布日期:2023-02-06
通讯作者:
李亚红
基金资助:
Yan Dang, Chaohong Jia, Yalan Wang, Li Wang, Yafei Li, Yahong Li( )
)
Received:2022-11-29
Revised:2023-01-04
Published:2023-02-06
Contact:
Yahong Li
Supported by:文章分享

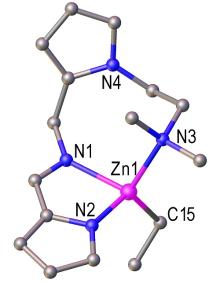
用ZnEt2分别与两个含吡咯基的配体2-(2-((((1H-吡咯-2-基)亚甲基)氨基)甲基)-1H-吡咯-1-基)-N,N-二甲基乙烷-1-胺(HL1)和N-((1H-吡咯-2-甲基)甲基)-1-(1H-吡啶-2-基)甲亚胺(H2L2)反应, 合成了两个化合物[Zn(L1)Et] (1)和[Zn2(L2)2(THF)2] (2). 通过核磁共振波谱、元素分析和单晶X射线衍射对配合物进行了表征. 研究了配合物1和2对芳基碘与B2Pin2 (B2Pin2=4,4,4',4',5,5,5',5'-八甲基-2,2′-双(1,3,2-二氧杂硼烷))偶联反应的催化作用. 它们都对这类硼化反应有催化活性. 化合物1显示出比2更高的活性. 化合物1催化的这类硼化反应具有温和的条件、宽的底物范围和较好的官能团相容性的特点. 此外, 还研究了先前报道的两种已知化合物[Li2(L1)2] (3)和[Mg(L1)2(THF)2] (4)对频哪硼烷(HBpin)和醛、酮的硼氢化反应的催化作用. 配合物3和4对该类反应具有较好的催化活性, 在很短的时间内以优异的产率生成了一系列硼酸酯.
党燕, 贾朝红, 王亚兰, 王丽, 李亚飞, 李亚红. 含吡咯基配体的锌、锂和镁配合物的合成与表征及其对芳基碘代物的硼化反应和醛、酮的硼氢化反应的催化作用[J]. 有机化学, 2023, 43(3): 1124-1135.
Yan Dang, Chaohong Jia, Yalan Wang, Li Wang, Yafei Li, Yahong Li. Synthesis and Characterization of Zinc, Lithium and Magnesium Complexes Containing Pyrrolyl Ligands, and Utilization as Catalysts in Borylation of Aryl Iodides and Hydroboration of Aldehydes and Ketones[J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1124-1135.



| Entry | Cat. (mol%) | Base | Time/h | Temp./℃ | Solvent | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | 1 (10) | KOEt | 12 | 75 | THF | >99 |
| 2 | 2 (10) | KOEt | 12 | 75 | THF | 54 |
| 3 | 1 (1) | KOEt | 12 | 75 | THF | 50 |
| 4 | 2 (1) | KOEt | 12 | 75 | THF | 66 |
| 5 | 1 (5) | KOEt | 12 | 60 | THF | 31 |
| 6 | 1 (5) | KOEt | 6 | 75 | THF | 51 |
| 7 | 3c (10) | KOEt | 12 | 75 | THF | 0 |
| 8 | 4c (10) | KOEt | 12 | 75 | THF | 0 |
| 9 | nBuLi | NaOEt | 12 | 75 | THF | 0 |
| 10 | nBu2Mg | NaOEt | 12 | 75 | THF | 0 |
| 11 | Et2Zn | NaOEt | 12 | 75 | THF | 0 |
| Entry | Cat. (mol%) | Base | Time/h | Temp./℃ | Solvent | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | 1 (10) | KOEt | 12 | 75 | THF | >99 |
| 2 | 2 (10) | KOEt | 12 | 75 | THF | 54 |
| 3 | 1 (1) | KOEt | 12 | 75 | THF | 50 |
| 4 | 2 (1) | KOEt | 12 | 75 | THF | 66 |
| 5 | 1 (5) | KOEt | 12 | 60 | THF | 31 |
| 6 | 1 (5) | KOEt | 6 | 75 | THF | 51 |
| 7 | 3c (10) | KOEt | 12 | 75 | THF | 0 |
| 8 | 4c (10) | KOEt | 12 | 75 | THF | 0 |
| 9 | nBuLi | NaOEt | 12 | 75 | THF | 0 |
| 10 | nBu2Mg | NaOEt | 12 | 75 | THF | 0 |
| 11 | Et2Zn | NaOEt | 12 | 75 | THF | 0 |
| Entry | Reactant | Time/h | Cat. (mol%) | Product | Yield/% |
|---|---|---|---|---|---|
| 1 | | 12 | 1 (5) | | 82b |
| 2 | | 12 | 2 (10) | | 43b |
| 3 | | 12 | 1 (10) | | 75c |
| 4 | | 12 | 2 (10) | | 64b |
| 5 | | 12 | 1 (10) | | 64c |
| Entry | Reactant | Time/h | Cat. (mol%) | Product | Yield/% |
| 6 | | 12 | 2 (10) | | 83b |
| 7 | | 12 | 1 (10) | | 89c |
| 8 | | 12 | 2 (10) | | 65b |
| 9 | | 12 | 1 (10) | | 71c |
| 10 | | 12 | 2 (10) | | 44b |
| 11 | | 12 | 1 (5) | | 99b |
| 12 | | 12 | 2 (10) | | 71b |
| 13 | | 12 | 1 (5) | — | NP |
| 14 | | 12 | 2 (10) | — | NP |
| 15 | | 12 | 1 (10) | | 63c |
| 16 | | 12 | 1 (10) | | 75c |
| Entry | Reactant | Time/h | Cat. (mol%) | Product | Yield/% |
| 17 | | 12 | 1 (10) | | 41b |
| 18 | | 12 | 1 (10) | | 39c |
| 19 | | 12 | 1 (10) | — | NPd |
| 20 | | 12 | 1 (10) | — | NPd |
| Entry | Reactant | Time/h | Cat. (mol%) | Product | Yield/% |
|---|---|---|---|---|---|
| 1 | | 12 | 1 (5) | | 82b |
| 2 | | 12 | 2 (10) | | 43b |
| 3 | | 12 | 1 (10) | | 75c |
| 4 | | 12 | 2 (10) | | 64b |
| 5 | | 12 | 1 (10) | | 64c |
| Entry | Reactant | Time/h | Cat. (mol%) | Product | Yield/% |
| 6 | | 12 | 2 (10) | | 83b |
| 7 | | 12 | 1 (10) | | 89c |
| 8 | | 12 | 2 (10) | | 65b |
| 9 | | 12 | 1 (10) | | 71c |
| 10 | | 12 | 2 (10) | | 44b |
| 11 | | 12 | 1 (5) | | 99b |
| 12 | | 12 | 2 (10) | | 71b |
| 13 | | 12 | 1 (5) | — | NP |
| 14 | | 12 | 2 (10) | — | NP |
| 15 | | 12 | 1 (10) | | 63c |
| 16 | | 12 | 1 (10) | | 75c |
| Entry | Reactant | Time/h | Cat. (mol%) | Product | Yield/% |
| 17 | | 12 | 1 (10) | | 41b |
| 18 | | 12 | 1 (10) | | 39c |
| 19 | | 12 | 1 (10) | — | NPd |
| 20 | | 12 | 1 (10) | — | NPd |
| Entry | Catalyst | Amt (mol%) | Time/min | Yieldb/% |
|---|---|---|---|---|
| 1 | 3 | 10 | 120 | >99 |
| 2 | 4 | 10 | 120 | >99 |
| 3 | 3 | 10 | 60 | >99 |
| 4 | 4 | 10 | 60 | >99 |
| 5 | 3 | 10 | 30 | >99 |
| 6 | 4 | 10 | 30 | >99 |
| 7 | 3 | 5 | 30 | >99 |
| 8 | 4 | 5 | 30 | >99 |
| 9 | 3 | 1 | 30 | >99 |
| 10 | 4 | 1 | 30 | >99 |
| Entry | Catalyst | Amt (mol%) | Time/min | Yieldb/% |
|---|---|---|---|---|
| 1 | 3 | 10 | 120 | >99 |
| 2 | 4 | 10 | 120 | >99 |
| 3 | 3 | 10 | 60 | >99 |
| 4 | 4 | 10 | 60 | >99 |
| 5 | 3 | 10 | 30 | >99 |
| 6 | 4 | 10 | 30 | >99 |
| 7 | 3 | 5 | 30 | >99 |
| 8 | 4 | 5 | 30 | >99 |
| 9 | 3 | 1 | 30 | >99 |
| 10 | 4 | 1 | 30 | >99 |
| Entry | Reactant | Time/min | Cat. | Product | Yieldb/% |
|---|---|---|---|---|---|
| 1 | | 30 | 3 | | >99 |
| 2 | | 30 | 4 | | >99 |
| 3 | | 30 | 3 | | — |
| 4 | | 30 | 4 | | >99 |
| 5 | | 30 | 3 | | >99 |
| 6 | | 30 | 4 | | 64 |
| 7 | | 30 | 3 | | >99 |
| Entry | Reactant | Time/min | Cat. | Product | Yieldb/% |
| 8 | | 30 | 4 | | >99 |
| 9 | | 30 | 3 | | >99 |
| 10 | | 30 | 4 | | >99 |
| Entry | Reactant | Time/min | Cat. | Product | Yieldb/% |
|---|---|---|---|---|---|
| 1 | | 30 | 3 | | >99 |
| 2 | | 30 | 4 | | >99 |
| 3 | | 30 | 3 | | — |
| 4 | | 30 | 4 | | >99 |
| 5 | | 30 | 3 | | >99 |
| 6 | | 30 | 4 | | 64 |
| 7 | | 30 | 3 | | >99 |
| Entry | Reactant | Time/min | Cat. | Product | Yieldb/% |
| 8 | | 30 | 4 | | >99 |
| 9 | | 30 | 3 | | >99 |
| 10 | | 30 | 4 | | >99 |
| Entry | Reactant | Time/min | Cat. | Product | Yieldb/% |
|---|---|---|---|---|---|
| 1 | | 30 | 3 | | >99 |
| 2 | | 30 | 4 | | >99 |
| 3 | | 30 | 3 | | >99 |
| 4 | | 30 | 4 | | >99 |
| 5 | | 30 | 3 | | >99 |
| 6 | | 30 | 4 | | >99 |
| Entry | Reactant | Time/min | Cat. | Product | Yieldb/% |
| 7 | | 30 | 3 | | >99 |
| 8 | | 30 | 4 | | >99 |
| 9 | | 30 | 3 | | >99 |
| 10 | | 30 | 4 | | >99 |
| Entry | Reactant | Time/min | Cat. | Product | Yieldb/% |
|---|---|---|---|---|---|
| 1 | | 30 | 3 | | >99 |
| 2 | | 30 | 4 | | >99 |
| 3 | | 30 | 3 | | >99 |
| 4 | | 30 | 4 | | >99 |
| 5 | | 30 | 3 | | >99 |
| 6 | | 30 | 4 | | >99 |
| Entry | Reactant | Time/min | Cat. | Product | Yieldb/% |
| 7 | | 30 | 3 | | >99 |
| 8 | | 30 | 4 | | >99 |
| 9 | | 30 | 3 | | >99 |
| 10 | | 30 | 4 | | >99 |
| [1] |
Specklin, D.; Fliedel, C.; Dagorne, S. Chem. Rec. 2021, 21, 1130.
doi: 10.1002/tcr.v21.5 |
| [2] |
Mannarsamy, M.; Nandeshwar, M.; Muduli, G.; Prabusankar, G. Chem. Asian J. 2022, 17, e202200594.
|
| [3] |
Hua, Y. Z.; Han, X. W.; Yang, X. C.; Song, X. X.; Wang, M. C.; Chang, J. B. J. Org. Chem. 2014, 79, 11690.
doi: 10.1021/jo5023712 |
| [4] |
Jia, Y.; Yang, W.; Du, D. M. Org. Biol. Chem. 2012, 10, 4739.
doi: 10.1039/c2ob25360g |
| [5] |
Shin, M.; Kim, M.; Hwang, C.; Lee, H.; Kwon, H.; Park, J.; Lee, E.; Cho, S. H. Org. Lett. 2020, 22, 2476.
doi: 10.1021/acs.orglett.0c00721 |
| [6] |
Procter, R. J.; Uzelac, M.; Cid, J.; Rushworth, P. J.; Ingleson, M. J. ACS Catal. 2019, 9, 5760.
doi: 10.1021/acscatal.9b01370 |
| [7] |
Dagorne, S. Synthesis 2018, 50, 3662.
doi: 10.1055/s-0037-1610088 |
| [8] |
Lortie, J. L.; Dudding, T.; Gabidullin, B. M.; Nikonov, G. I. ACS Catal. 2017, 7, 8454.
doi: 10.1021/acscatal.7b02811 |
| [9] |
Ataie, S.; Ovens, J. S.; Baker, R. T. Chem. Commun. 2022, 58, 8266.
doi: 10.1039/D2CC03517K |
| [10] |
Jaiswal, K.; Groutchik, K.; Bawari, D.; Dobrovetsky, R. ChemCatChem 2022, 14, e202200004.
|
| [11] |
Grundy, M. E.; Yuan, K.; Nichol, G. S.; Ingleson, M. J. Chem. Sci. 2021, 12, 8190.
doi: 10.1039/D1SC01883C |
| [12] |
Uzelac, M.; Yuan, K.; Ingleson, M. J. Organometallics 2020, 39, 1332.
doi: 10.1021/acs.organomet.0c00086 |
| [13] |
Cruz-Martínez, F. D. L.; Buchaca, M. M. D. S.; Fernández-Baeza, J.; Sánchez-Barba, L. F.; Rodríguez, A. M.; Castro-Osma, J. A.; Lara-Sánchez, A. Inorg. Chem. 2021, 60, 532.
|
| [14] |
Colonna, P.; Bezzenine, S.; Gil, R.; Hannedouche, J. Adv. Synth. Catal. 2020, 362, 1550.
doi: 10.1002/adsc.v362.8 |
| [15] |
Garden, J. A.; White, A. J. P.; Williams, C. K. Dalton Trans. 2017, 46, 2532.
doi: 10.1039/C6DT04193K |
| [16] |
Bazzicalupi, C.; Bencini, A.; Berni, E.; Vaira, M. D. Inorg. Chim. Acta 2005, 358, 77.
doi: 10.1016/j.ica.2004.07.018 |
| [17] |
Trost, B. M.; Hitce, J. J. Am. Chem. Soc. 2009, 131, 4572.
doi: 10.1021/ja809723u |
| [18] |
Walker, D. A.; Woodman, T. J.; Schormann, M.; Hughes, D. L.; Bochmann, M. Organometallics 2003, 22, 797.
doi: 10.1021/om020690a |
| [19] |
Bose, S. K.; Deißenberger, A.; Eichhorn, A.; Steel, P. G.; Lin, Z.; Marder, T. B. Angew. Chem., Int. Ed. 2015, 54, 11843.
doi: 10.1002/anie.v54.40 |
| [20] |
Bose, S. K.; Marder, T. B. Org. Lett. 2014, 16, 4562.
doi: 10.1021/ol502120q |
| [21] |
Bose, S. K.; Fucke, K.; Liu, L.; Steel, P. G.; Marder, T. B. Angew. Chem., Int. Ed. 2014, 53, 1799.
doi: 10.1002/anie.201308855 |
| [22] |
Nagashima, Y.; Takita, R.; Yoshida, K.; Hirano, K.; Uchiyama, M. J. Am. Chem. Soc. 2013, 135, 18730.
doi: 10.1021/ja409748m pmid: 24266767 |
| [23] |
Campos, J.; Aldridge, S. Angew. Chem., Int. Ed. 2015, 54, 14159.
doi: 10.1002/anie.201507627 pmid: 26411884 |
| [24] |
Zhang, L.; Jiao, L. J. Am. Chem. Soc. 2019, 141, 9124.
doi: 10.1021/jacs.9b00917 pmid: 31140798 |
| [25] |
Cheng, Y.; Muck-Lichtenfeld, C.; Studer, A. Angew. Chem., Int. Ed. 2018, 57, 16832.
doi: 10.1002/anie.201810782 pmid: 30332527 |
| [26] |
McCarty, B. J.; Tang, W. P. Green Synthesis and Catalysis 2021, 2, 1.
doi: 10.1016/j.gresc.2020.12.004 |
| [27] |
Zhu, S. X.; Yan, J. X.; Zhou, Y.; Yang, K.; Song, Q. L. Green Synth. Catal. 2021, 2, 299.
|
| [28] |
Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed.; Ed.: Hall, D. G., Wiley-VCH, Weinheim, Germany, 2011.
|
| [29] |
Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
doi: 10.1021/cr00039a007 |
| [30] |
Budiman, Y. P.; Westcott, S. A.; Radius, U.; Marder, T. B. Adv. Synth. Catal. 2021, 363, 2224.
doi: 10.1002/adsc.v363.9 |
| [31] |
Brown, H. C. Organic Synthesis via Boranes, Wiley-Inter-science, New York, 1975.
|
| [32] |
Chow, W. K.; Yuen, O. Y.; Choy, P. Y.; So, C. M.; Lau, C. P.; Wong, W. T.; Kwong, F. Y. RSC Adv. 2013, 3, 12518.
doi: 10.1039/c3ra22905j |
| [33] |
Budiman, Y. P.; Lorenzen, S.; Liu, Z. Q.; Radius, U.; Marder, T. B. Chem.-Eur. J. 2021, 27, 3869.
doi: 10.1002/chem.202004648 pmid: 33197081 |
| [34] |
Kleeberg, C.; Dang, L.; Lin, Z. Y.; Marder, T. B. Angew. Chem.,Int. Ed. 2009, 48, 5350.
doi: 10.1002/anie.200901879 |
| [35] |
Li, Y. F.; Dang, Y.; Li, D. W.; Pan, H. F.; Zhang, L. Wang, L.; Cao, Z.; Li, Y. H. Organometallics 2021, 40, 482.
doi: 10.1021/acs.organomet.0c00733 |
| [36] |
Zhang, L.; Li, Y. F.; Wang, L.; Cao, Z.; Zhang, Q.; Li, Y. H. Eur. J. Inorg. Chem. 2022, 2022, e202200079.
|
| [37] |
Wu, M. C.; Hu, T. C.; Lo, Y. C.; Lee, T. Y.; Lin, C. H.; Lu, W. Y.; Lin, C. C.; Datta, A.; Huang, J. H. J. Organomet. Chem. 2015, 791, 141.
doi: 10.1016/j.jorganchem.2015.05.006 |
| [38] |
Hsiao, C. S.; Wang, T. Y.; Datta, A.; Liao, F. X.; Hu, C. H.; Lin, C. H.; Huang, J. H.; Lee, T. Y. J. Organomet. Chem. 2012, 718, 82.
doi: 10.1016/j.jorganchem.2012.08.016 |
| [39] |
Loke, S. K.; Pagadala, E.; Devaraju, S.; Srinivasadesikan, V.; Kottalanka, R. K. RSC Adv. 2020, 10, 36275.
doi: 10.1039/D0RA07837A |
| [40] |
Zheng, X. X.; Wang, Z. X. J. Organomet. Chem. 2016, 823, 14.
doi: 10.1016/j.jorganchem.2016.09.002 |
| [41] |
Kong, W. L.; Wang, Z. X. Dalton Trans. 2014, 43, 9126.
doi: 10.1039/c4dt00431k |
| [42] |
Vignesh Babu, H.; Muralidharan, K. Dalton Trans. 2013, 42, 1238.
doi: 10.1039/C2DT31755A |
| [43] |
D’Auria, I.; Tedesco, C.; Mazzeo, M.; Pellecchia, C. Dalton Trans. 2017, 46, 12217.
doi: 10.1039/C7DT02445B |
| [44] |
Alonso de la Pena, M.; Merzoud, L.; Lamine, W.; Tuel, A.; Chermette, H.; Christ, L. J. CO2 Util. 2021, 44, 101380.
|
| [45] |
Chen, J. J.; Xu, Y. C.; Gan, Z. L.; Peng, X.; Yi, X. Y. Eur. J. Inorg. Chem. 2019, 147, 1733.
|
| [46] |
Mercade, E.; Zangrando, E.; Claver, C.; Godard, C. ChemCatChem. 2016, 8, 234.
doi: 10.1002/cctc.201500772 |
| [47] |
Liu, Q.; Guo, Z. Q.; Han, H. F.; Tong, H. B.; Wei, X. H. Polyhedron 2015, 85, 15.
doi: 10.1016/j.poly.2014.08.009 |
| [48] |
Dang, Y.; Wang, Y. L.; Li, Y. F.; Xu, M.; Jia, C. H.; Lu, Y. H.; Zhang, L.; Li, Y. H.; Xia, Y. Z. Organometallics 2021, 40, 1830.
doi: 10.1021/acs.organomet.0c00815 |
| [49] |
Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. Chem.-Eur. J. 2005, 11, 1479.
pmid: 15657963 |
| [50] |
Li, Y. F.; Pan, H. F.; Lu, Y. H.; Luo, Y. S.; Dang, Y.; Wang, Y. L.; Xia, S. W.; Li, Y. H.; Xia, Y. Z. Dalton Trans. 2022, 51, 3616.
doi: 10.1039/D1DT03235F |
| [51] |
Falconnet, A.; Magre, M.; Maity, B.; Cavallo, L.; Rueping, M. Angew. Chem., Int. Ed. 2019, 58, 17567.
doi: 10.1002/anie.201908012 pmid: 31642572 |
| [52] |
Mukherjee, D.; Shirase, S.; Spaniol, T. P.; Mashima, K.; Okuda, J. Chem. Commun. 2016, 52, 13155.
doi: 10.1039/C6CC06805G |
| [53] |
Bisai, M. K.; Das, T.; Vanka, K.; Sen, S. S. Chem. Commun. 2018, 54, 6843.
doi: 10.1039/C8CC02314J |
| [54] |
Dudnik, A. S.; Weidner, V. L.; Motta, A.; Delferro, M.; Marks, T. J. Nat. Chem. 2014, 6, 1100.
doi: 10.1038/nchem.2087 |
| [55] |
Harinath, A.; Bhattacharjee, J.; Nayek, H. P.; Panda, T. K. Dalton Trans. 2018, 47, 12613.
doi: 10.1039/C8DT02032A |
| [56] |
Wu, Y.; Shan, C.; Ying, J.; Su, J.; Zhu, J.; Liu, L. L.; Zhao, Y. Green Chem. 2017, 19, 4169.
doi: 10.1039/C7GC01632H |
| [57] |
Weidner, V. L.; Barger, C. J.; Delferro, M.; Lohr, T. L.; Marks, T. J. ACS Catal. 2017, 7, 1244.
doi: 10.1021/acscatal.6b03332 |
| [58] |
Yadav, S.; Pahar, S.; Sen, S. S. Chem. Commun. 2017, 53, 4562.
doi: 10.1039/C7CC02311A |
| [59] |
Chen, S.; Yan, D.; Xue, M.; Hong, Y.; Yao, Y.; Shen, Q. Org. Lett. 2017, 19, 3382.
doi: 10.1021/acs.orglett.7b01335 |
| [60] |
Eedugurala, N.; Wang, Z.; Chaudhary, U.; Nelson, N.; Kandel, K.; Kobayashi, T.; Slowing, I. I.; Pruski, M; Sadow, A. D. ACS Catal. 2015, 5, 7399.
doi: 10.1021/acscatal.5b01671 |
| [61] |
Oluyadi, A. A.; Ma, S.; Muhoro, C. N. Organometallics 2013, 32, 70.
doi: 10.1021/om300828m |
| [62] |
Chong, C. C.; Hirao, H.; Kinjo, R. Angew. Chem., Int. Ed. 2015, 54, 190.
doi: 10.1002/anie.v54.1 |
| [1] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [2] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [3] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [4] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [5] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [6] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [7] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [8] | 李洋, 董亚楠, 李跃辉. 经由N-硼基酰胺中间体的酰胺高效转化合成腈类化合物[J]. 有机化学, 2024, 44(2): 638-643. |
| [9] | 李思达, 崔鑫, 舒兴中, 吴立朋. 钛催化的烯烃制备1,1-二硼化合物[J]. 有机化学, 2024, 44(2): 631-637. |
| [10] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [11] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [12] | 李梦竹, 孟博莹, 兰文捷, 傅滨. 邻亚甲醌与硫叶立德反应合成2,3-二取代苯并二氢呋喃化合物[J]. 有机化学, 2024, 44(1): 195-203. |
| [13] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [14] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [15] | 文思, 丁宇浩, 田青于, 葛进, 程国林. 铑(III)催化苯甲亚胺酸乙酯和CF3-亚胺氧锍叶立德C—H 活化/环化反应合成CF3-1H-苯并[de][1,8]萘吡啶[J]. 有机化学, 2024, 44(1): 291-300. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||