有机化学 ›› 2023, Vol. 43 ›› Issue (3): 961-973.DOI: 10.6023/cjoc202211045 上一篇 下一篇
所属专题: 中国女科学家专辑
综述与进展
收稿日期:2022-11-30
修回日期:2023-02-03
发布日期:2023-02-14
通讯作者:
刘小华
基金资助:Received:2022-11-30
Revised:2023-02-03
Published:2023-02-14
Contact:
Xiaohua Liu
Supported by:文章分享
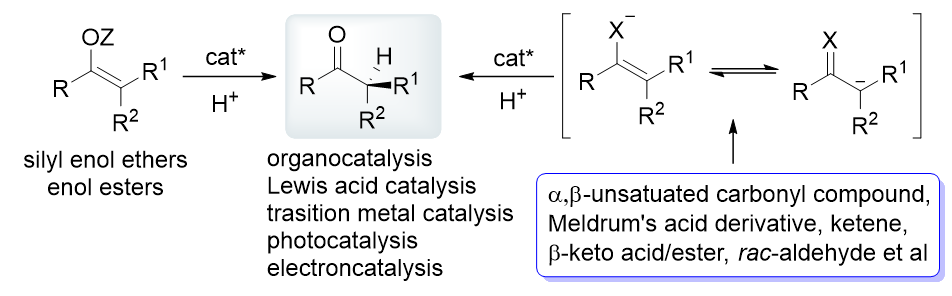
不对称催化质子化反应是构建含有α-叔碳立体中心手性羰基化合物最直接、有效的方法, 被广泛用于有机合成和药物化学中. 近几十年, 该领域发展迅速, 基于底物种类、催化体系和反应类型的相关综述相继被报道. 更新并总结了该领域2019年以来的重要进展, 主要通过烯醇或其衍生物为底物的直接不对称质子化和原位生成烯醇化物/α-碳负离子或自由基的不对称串联质子化这两种反应方式介绍相关工作, 以底物种类为主线, 穿插不同催化体系、活化模式以及催化策略和手段. 期望通过这些研究为化学研究者们提供思路, 进一步促进羰基化学和不对称催化等领域的发展, 为天然产物及其中间体、药物、候选药物等具有高附加值手性分子的简单、高效合成提供助力.
曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973.
Weidi Cao, Xiaohua Liu. Recent Advances on Catalytic Enantioselective Protonation for Construction of α-Tertiary Carbonyl Compounds[J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 961-973.
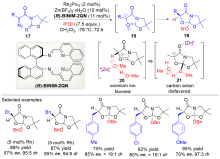

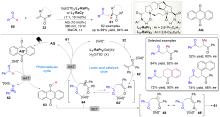

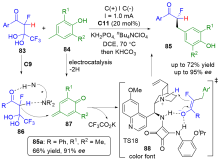
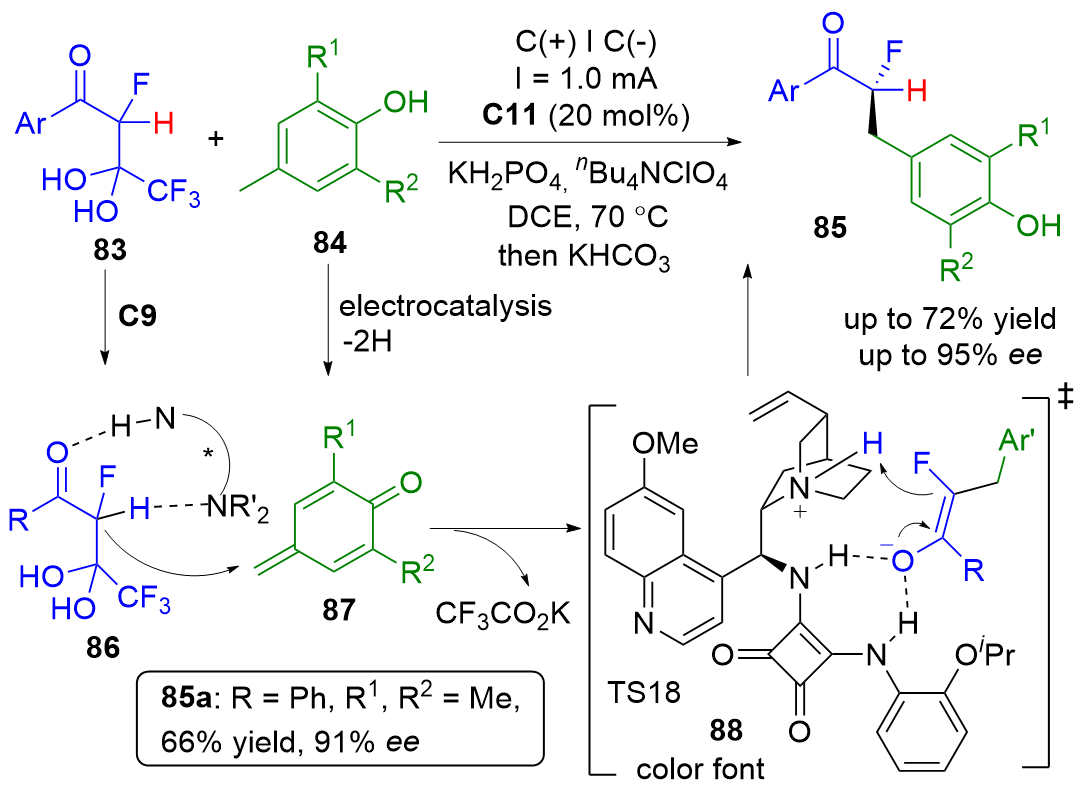
| [1] |
Marckwald, W. Ber. Dtsch. Chem. Ges. 1904, 37, 349.
doi: 10.1002/cber.v37:1 |
| [2] |
Marckwald, W. Ber. Dtsch. Chem. Ges. 1904, 37, 1368.
doi: 10.1002/cber.v37:2 |
| [3] |
Pracejus, H. Justus Liebigs Ann. Chem. 1960, 634, 9.
doi: 10.1002/(ISSN)1099-0690 |
| [4] |
Fehr, C. Angew. Chem., nt. Ed. 1996, 35, 2566.
|
| [5] |
Eames, J.; Weerasooriya, N. Tetrahedron: Asymmetry 2001, 12, 1.
doi: 10.1016/S0957-4166(00)00496-1 |
| [6] |
Duhamel, L.; Duhamel, P.; Plaquevent, J.-C.; Tetrahedron: Asymmetry 2004, 15, 3653.
doi: 10.1016/j.tetasy.2004.09.035 |
| [7] |
Blanchet, J.; Baudoux, J.; Amere, M.; Lasne, M.-C.; Rouden, J. Eur. J. Org. Chem. 2008, 5493.
|
| [8] |
Mohr, J. T.; Hong, A. Y.; Stoltz, B. M. Nat. Chem. 2009, 1, 359.
doi: 10.1038/nchem.297 |
| [9] |
Claraz, A.; Oudeyer, S.; Levacher, V. Curr. Org. Chem. 2012, 16, 2192.
doi: 10.2174/138527212803520308 |
| [10] |
Oudeyer, S.; Brière, J.-F.; Levacher, V. Eur. J. Org. Chem. 2014, 6103.
|
| [11] |
Phelan, J. P.; Ellman, J. A. Beilstein J. Org. Chem. 2016, 12, 1203.
doi: 10.3762/bjoc.12.116 pmid: 27559372 |
| [12] |
Legros, F.; Oudeyer, S.; Levacher, V. Chem. Rec. 2017, 17, 429.
doi: 10.1002/tcr.v17.4 |
| [13] |
Fu, N.; Zhang, L.; Luo, S. Org. Biomol. Chem. 2018, 16, 510.
doi: 10.1039/C7OB02615C |
| [14] |
Kingston, C.; James, J.; Guiry, P. J. J. Org. Chem. 2019, 84, 473.
doi: 10.1021/acs.joc.8b02478 pmid: 30376624 |
| [15] |
Liao, K.; Hu, X.-S.; Zhu, R.-Y.; Rao, R.-H.; Yu, J.-S.; Zhou, F.; Zhou, J. Chin. J. Chem. 2019, 37, 799.
doi: 10.1002/cjoc.v37.8 |
| [16] |
Łowicki, D.; Watral, J.; Jelecki, M.; Bohusz, W.; Kwit, M. Tetrahedron 2021, 86, 132085.
doi: 10.1016/j.tet.2021.132085 |
| [17] |
Yamamoto, E.; Wakafuji, K.; Mori, Y.; Teshima, G.; Hidani, Y.; Tokunaga, M. Org. Lett. 2019, 21, 4030.
doi: 10.1021/acs.orglett.9b01216 pmid: 31124363 |
| [18] |
Mandrelli, F.; Blond, A.; James, T.; Kim, H.; List, B. Angew. Chem., Int. Ed. 2019, 58, 11479.
doi: 10.1002/anie.v58.33 |
| [19] |
Martzel, T.; Annibaletto, J.; Levacher, V.; Brière, J.-F.; Oudeyera, S. Adv. Synth. Catal. 2019, 361, 995.
doi: 10.1002/adsc.201801453 |
| [20] |
Legros, F.; Martzel, T.; Brière, J.-F.; Oudeyer, S.; Levacher, V. Eur. J. Org. Chem. 2018, 1975.
|
| [21] |
Toda, Y.; Yoshida, T.; Arisue, K.; Fukushima, K.; Esaki, H.; Kikuchi, A.; Suga, H. Chem.-Eur. J. 2021, 27, 10578.
doi: 10.1002/chem.v27.41 |
| [22] |
Wang, Q.-Y.; Liu, T.-F.; Chu, L.-F.; Yao, Y.; Lu, C.-D. Chem. Commun. 2021, 57, 11992.
doi: 10.1039/D1CC05307H |
| [23] |
Park, S. J.; Hwang, I.-S.; Chang, Y. J.; Song, C. E. J. Am. Chem. Soc. 2021, 143, 2552.
doi: 10.1021/jacs.0c11815 |
| [24] |
Narayan, S.; Muldoon, J.; Finn., M. G. Fokin, V. V.; Kolb, H. C.; Sharpless, K. B. Angew. Chem., Int. Ed. 2005, 44, 3275.
doi: 10.1002/(ISSN)1521-3773 |
| [25] |
Li, Y.-P.; Li, Z.-Q.; Zhou, B.; Li, M.-L.; Xue, X.-S.; Zhu, S.-F.; Zhou, Q.-L. ACS Catal. 2019, 9, 6522.
doi: 10.1021/acscatal.9b01502 |
| [26] |
Xu, B.; Zhu, S.-F.; Xie, X.-L.; Shen, J.-J.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2011, 50, 11483.
doi: 10.1002/anie.v50.48 |
| [27] |
Li, Y.-P.; Zhu, S.-F.; Zhou, Q.-L. Org. Lett. 2019, 21, 9391.
doi: 10.1021/acs.orglett.9b03615 |
| [28] |
Azuma, T.; Murata, A.; Kobayashi, Y.; Inokuma, T.; Takemoto, Y. Org. Lett. 2014, 16, 4256.
doi: 10.1021/ol501954r |
| [29] |
Hayama, N.; Földes, T.; Nishibayashi, K.; Kuramoto, R.; Kobayashi, Y.; Pápai, I.; Takemoto, Y. J. Am. Chem. Soc. 2018, 140, 12216.
doi: 10.1021/jacs.8b07511 |
| [30] |
Murakami, H.; Yamada, A.; Michigami, K.; Takemoto, Y. Asian J. Org. Chem. 2021, 10, 1097.
doi: 10.1002/ajoc.v10.5 |
| [31] |
Jian, J.-H.; Zeng, H.-W.; Kuo, T.-S.; Wu, P.-Y.; Wu, H.-L. Org. Lett. 2019, 21, 9468.
doi: 10.1021/acs.orglett.9b03666 pmid: 31742418 |
| [32] |
Chen, J.-P.; Xu, M.-H. Org. Biomol. Chem. 2020, 18, 4569.
doi: 10.1039/D0OB00616E |
| [33] |
Chen, J.-P.; Li, Y.; Liu, C.; Wang, T.; Chung, L. W.; Xu, M.-H. Org. Lett. 2021, 23, 571.
doi: 10.1021/acs.orglett.0c04099 |
| [34] |
Shibatomi, K.; Kitahara, K.; Sasaki, N.; Fuzisawa, I.; Iwasa, S. Nat. Commun. 2017, 8, 15600.
doi: 10.1038/ncomms15600 pmid: 28580951 |
| [35] |
Mizutani, H.; Kawanishi, R.; Shibatomi, K. Chem. Commun. 2021, 57, 6676.
doi: 10.1039/D1CC01610E |
| [36] |
Han, Y.; Zhang, L.; Luo, S. Org. Lett. 2019, 21, 7258.
doi: 10.1021/acs.orglett.9b02491 |
| [37] |
Han, Y.; Zhang, L.; Luo, S. Org. Lett. 2022, 24, 1752.
doi: 10.1021/acs.orglett.2c00435 |
| [38] |
Han, Y.; Shi, M.; Mi, X.; Luo, S. Chem.-Eur. J. 2022, 28, e202202584.
|
| [39] |
Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898.
doi: 10.1021/acs.joc.6b01449 |
| [40] |
Stephenson, C. R. J.; Yoon, T. P.; MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry, Weinheim, Germany, 2018.
|
| [41] |
Melchiorre, P. Chem. Rev. 2022, 122, 1483.
doi: 10.1021/acs.chemrev.1c00993 pmid: 35078320 |
| [42] |
Yoshida, J.-I.; Kataoka, K.; Horcajada, R.; Nagaki, A. Chem. Rev. 2008, 108, 2265
doi: 10.1021/cr0680843 |
| [43] |
Wiebe, A.; Gieshoff, T.; Mçhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2018, 57, 5594.
doi: 10.1002/anie.201711060 |
| [44] |
Dai, Z.-Y.; Nong, Z.-S.; Wang, P.-S. ACS Catal. 2020, 10, 4786.
doi: 10.1021/acscatal.0c00610 |
| [45] |
Dai, Z.-Y.; Nong, Z.-S.; Song, S.; Wang, P.-S. Org. Lett. 2021, 23, 3157.
doi: 10.1021/acs.orglett.1c00801 |
| [46] |
Cheng, X. L.; Li, D.; Yang, B. X.; Lin, Y.-M.; Gong, L. Chin. J. Org. Chem. 2022, 42, 3335. (in Chinese)
doi: 10.6023/cjoc202205032 |
|
(程秀亮, 李冬, 杨博轩, 林玉妹, 龚磊, 有机化学, 2022, 42, 3335.)
|
|
| [47] |
Luo, Y.; Wei, Q.; Yang, L. K.; Zhou, Y. Q.; Cao, W. D.; Su, Z. S.; Liu, X. H.; Feng, X. M. ACS Catal. 2022, 12, 12984.
doi: 10.1021/acscatal.2c04047 |
| [48] |
Liu, X. H.; Lin, L. L.; Feng, X. M. Acc. Chem. Res. 2011, 44, 574.
doi: 10.1021/ar200015s |
| [49] |
Liu, X. H.; Lin, L. L.; Feng, X. M. Org. Chem. Front. 2014, 1, 298.
doi: 10.1039/c3qo00059a |
| [50] |
Liu, X. H.; Zheng, H. F.; Xia, Y.; Lin, L. L.; Feng, X. M. Acc. Chem. Res. 2017, 50, 2621.
doi: 10.1021/acs.accounts.7b00377 |
| [51] |
Liu, X. H.; Dong, S. X.; Lin, L. L.; Feng, X. M. Chin. J. Chem. 2018, 36, 791.
doi: 10.1002/cjoc.v36.9 |
| [52] |
Wang, M. Y.; Li, W. Chin. J. Chem. 2021, 39, 969.
doi: 10.1002/cjoc.v39.4 |
| [53] |
Dong, S. X.; Liu, X. H.; Feng, X. M. Acc. Chem. Res. 2022, 55, 415.
doi: 10.1021/acs.accounts.1c00664 |
| [54] |
Zhan, T. Y.; Yang, L. K.; Chen, Q. Y.; Weng, R.; Liu, X. H.; Feng, X. M. CCS Chem. 2022, DOI: 10.31635/ccschem.022.202202405.
doi: 10.31635/ccschem.022.202202405 |
| [55] |
Zhao, Y.; Zhang, C.; Chin, K. F.; Pytela, O.; Wei, G.; Liu, H.; Bureš, F.; Jiang, Z. RSC Adv. 2014, 4, 30062.
doi: 10.1039/C4RA05525J |
| [56] |
Hou, M.; Lin, L.; Chai, X.; Zhao, X.; Qiao, B.; Jiang, Z. Chem. Sci. 2019, 10, 6629.
doi: 10.1039/C9SC02000D |
| [57] |
Kong, M.; Tan, Y.; Zhao, Xi.; Qiao, B.; Tan, C.-H.; Cao, S.; Jiang, Z. J. Am. Chem. Soc. 2021, 143, 4024.
doi: 10.1021/jacs.1c01073 |
| [58] |
Zhu, Y.; Zhang, L.; Luo, S. J. Am. Chem. Soc. 2016, 138, 3978.
doi: 10.1021/jacs.6b00627 |
| [59] |
Huang, M.; Zhang, L.; Pan, T.; Luo, S. Science 2022, 375, 869.
doi: 10.1126/science.abl4922 |
| [60] |
Gu, Z.; Zhang, L.; Li, H.; Cao, S.; Yin, Y.; Zhao, X.; Ban, X.; Jiang, Z. Angew. Chem., Int. Ed. 2022, 61, e202211241.
|
| [61] |
Chang, X.; Zhang, J.; Zhang, Q.; Guo, C. Angew. Chem., Int. Ed. 2020, 59, 18500.
doi: 10.1002/anie.v59.42 |
| [62] |
Mondal, S.; Dumur, F.; Gigmes, D.; Sibi, M. P.; Bertrand, M. P.; Nechab, M. Chem. Rev. 2022, 122, 5842.
doi: 10.1021/acs.chemrev.1c00582 |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [4] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [5] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [6] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [7] | 蒙玲, 汪君. 硫代黄烷酮类衍生物的合成研究进展[J]. 有机化学, 2023, 43(3): 873-891. |
| [8] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [9] | 陈志远, 杨梦维, 徐建林, 徐允河. 铜催化双炔膦氧化物硅质子化反应合成β-硅基取代的乙烯基膦氧化物[J]. 有机化学, 2023, 43(10): 3598-3607. |
| [10] | 曾燕, 叶飞. 不对称催化构建硅立体中心化合物的新反应体系研究进展[J]. 有机化学, 2023, 43(10): 3388-3413. |
| [11] | 赵佳怡, 葛怡聪, 何川. 不对称催化Si—H/X—H脱氢偶联构筑硅中心手性[J]. 有机化学, 2023, 43(10): 3352-3366. |
| [12] | 代增进, 张绪穆, 殷勤. 铵盐为胺源的不对称还原胺化反应研究进展[J]. 有机化学, 2022, 42(8): 2261-2274. |
| [13] | 李晖, 殷亮. 铜催化的直接型插烯反应研究进展[J]. 有机化学, 2022, 42(6): 1573-1585. |
| [14] | 尹艳丽, 赵筱薇, 江智勇. 可见光不对称催化合成手性氮杂芳烃衍生物[J]. 有机化学, 2022, 42(6): 1609-1625. |
| [15] | 吴逾诸, 申盼盼, 段文增, 马玉道. 卡宾催化对亚甲基苯醌的不对称硼化反应的研究[J]. 有机化学, 2022, 42(5): 1483-1492. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
