Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (2): 631-637.DOI: 10.6023/cjoc202309021 Previous Articles Next Articles
ARTICLES
李思达a,b,c, 崔鑫a, 舒兴中b,*( ), 吴立朋a,d,*(
), 吴立朋a,d,*( )
)
收稿日期:2023-09-21
修回日期:2023-10-04
发布日期:2023-10-12
作者简介:基金资助:
Sida Lia,b,c, Xin Cuia, Xing-Zhong Shub( ), Lipeng Wua,d(
), Lipeng Wua,d( )
)
Received:2023-09-21
Revised:2023-10-04
Published:2023-10-12
Contact:
E-mail: About author:Supported by:Share
Sida Li, Xin Cui, Xing-Zhong Shu, Lipeng Wu. Titanium-Catalyzed Synthesis of 1,1-Diborylalkanes from Aryl Alkenes[J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 631-637.

| Entry | Catalyst | Solvent | Yieldb/% | ||
|---|---|---|---|---|---|
| 3 | 4 | 5 | |||
| 1 | CpTiCl3 | Toluene | 3 | 28 | 18 |
| 2 | Cp*TiCl3 | Toluene | 14 | 41 | 34 |
| 3 | IndTiCl3 | Toluene | 0 | 56 | 2 |
| 4 | Cp2TiCl2 | Toluene | 24 | 30 | 46 |
| 5 | SiMe2Cp2TiCl2 | Toluene | 4 | 41 | 12 |
| 6 | Cp2TiCl2 | Dioxane | 8 | 56 | 36 |
| 7 | Cp2TiCl2 | MTBE | 8 | 25 | 32 |
| 8 | Cp2TiCl2 | THF | 9 | 71 | 20 |
| 9 | Cp2TiCl2 | Et2O | 8 | 24 | 68 |
| 10 | Cp2TiCl2 | Hexane | 9 | 25 | 66 |
| 11 | Cp2TiCl2 | Neat | 10 | 16 | 74 |
| Entry | Catalyst | Solvent | Yieldb/% | ||
|---|---|---|---|---|---|
| 3 | 4 | 5 | |||
| 1 | CpTiCl3 | Toluene | 3 | 28 | 18 |
| 2 | Cp*TiCl3 | Toluene | 14 | 41 | 34 |
| 3 | IndTiCl3 | Toluene | 0 | 56 | 2 |
| 4 | Cp2TiCl2 | Toluene | 24 | 30 | 46 |
| 5 | SiMe2Cp2TiCl2 | Toluene | 4 | 41 | 12 |
| 6 | Cp2TiCl2 | Dioxane | 8 | 56 | 36 |
| 7 | Cp2TiCl2 | MTBE | 8 | 25 | 32 |
| 8 | Cp2TiCl2 | THF | 9 | 71 | 20 |
| 9 | Cp2TiCl2 | Et2O | 8 | 24 | 68 |
| 10 | Cp2TiCl2 | Hexane | 9 | 25 | 66 |
| 11 | Cp2TiCl2 | Neat | 10 | 16 | 74 |
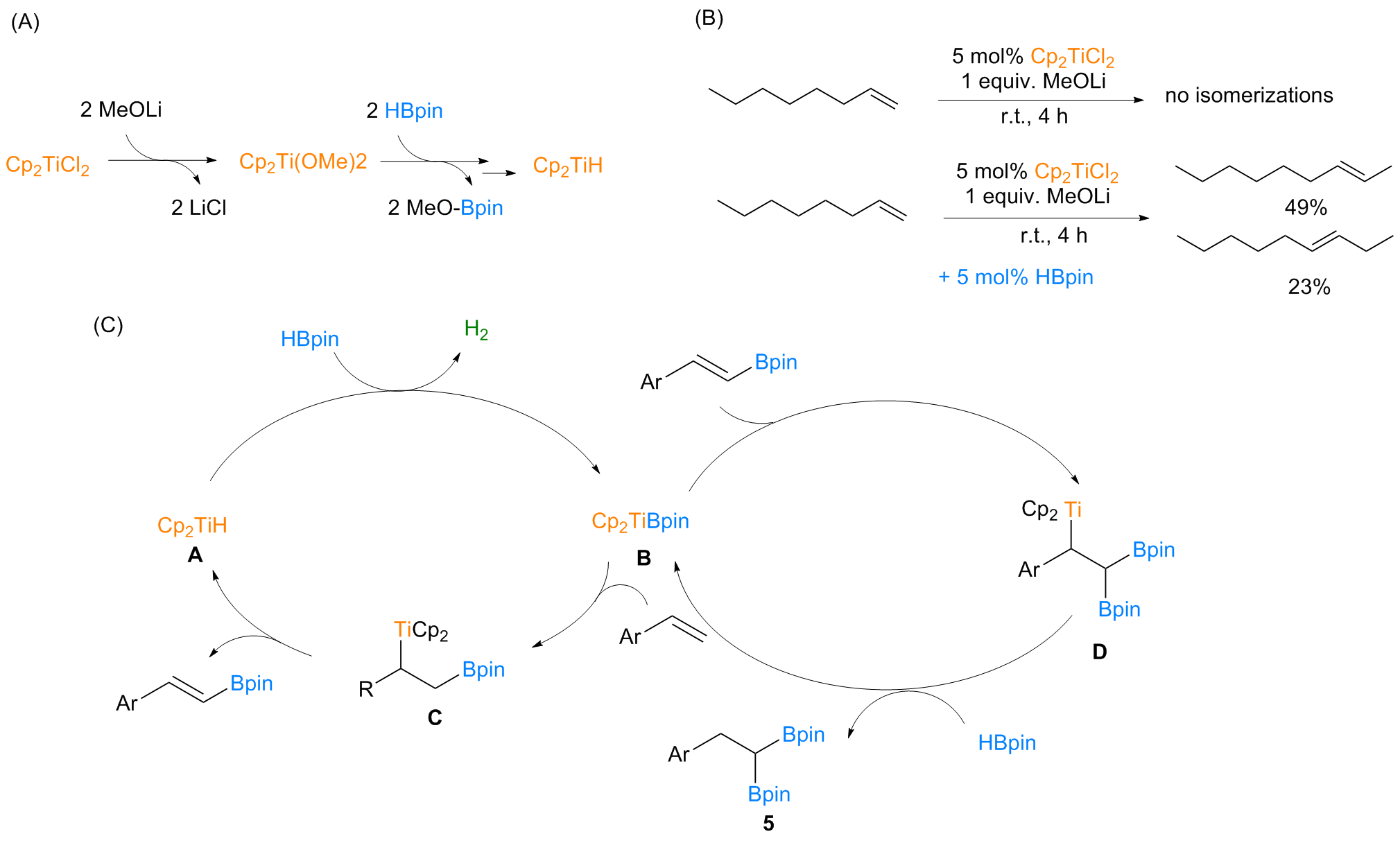
| [1] |
(a) Bonet, A.; Pubill-Ulldemolins, C.; Bo, C.; Gulyas, H.; Fernandez, E. Angew. Chem.. Int. Ed. 2011, 50, 7158.
doi: 10.1002/anie.v50.31 pmid: 31386387 |
|
(b) Zhang, L.; Huang, Z. J. Am. Chem. Soc. 2015, 137, 15600.
doi: 10.1021/jacs.5b11366 pmid: 31386387 |
|
|
(c) Morinaga, A.; Nagao, K.; Ohmiya, H.; Sawamura, M. Angew. Chem.. Int. Ed. 2015, 54, 15859.
doi: 10.1002/anie.v54.52 pmid: 31386387 |
|
|
(d) Zuo, Z. Q.; Huang, Z. Org. Chem. Front. 2016, 3, 434.
doi: 10.1039/C5QO00426H pmid: 31386387 |
|
|
(e) Li, C.; Wang, J.; Barton, L. M.; Yu, S.; Tian, M.; Peters, D. S.; Kumar, M.; Yu, A. W.; Johnson, K. A.; Chatterjee, A. K.; Yan, M.; Baran, P. S. Scienc. 2017, 356, eaam7355.
pmid: 31386387 |
|
|
(f) Hu, J.; Zhao, Y.; Shi, Z. Nat. Catal. 2018, 1, 860.
doi: 10.1038/s41929-018-0147-9 pmid: 31386387 |
|
|
(g) Lv, W.-X.; Li, Q.; Li, J.-L.; Li, Z.; Lin, E.; Tan, D.-H.; Cai, Y.-H.; Fan, W.-X.; Wang, H. Angew. Chem.. Int. Ed. 2018, 57, 16544.
doi: 10.1002/anie.v57.50 pmid: 31386387 |
|
|
(h) Liu, X.; Ming, W.; Zhang, Y.; Friedrich, A.; Marder, T. B. Angew. Chem.. Int. Ed. 2019, 58, 18923.
doi: 10.1002/anie.v58.52 pmid: 31386387 |
|
|
(i) Zhao, B.; Li, Z.; Wu, Y.; Wang, Y.; Qian, J.; Yuan, Y.; Shi, Z. Angew. Chem.. Int. Ed. 2019, 58, 9448.
doi: 10.1002/anie.v58.28 pmid: 31386387 |
|
|
(j) Yukimori, D.; Nagashima, Y.; Wang, C.; Muranaka, A.; Uchiyama, M. J. Am. Chem. Soc. 2019, 141, 9819.
doi: 10.1021/jacs.9b04665 pmid: 31386387 |
|
|
(k) Yamamoto, T.; Ishibashi, A.; Suginome, M. Org. Lett. 2019, 21, 6235.
doi: 10.1021/acs.orglett.9b02112 pmid: 31386387 |
|
|
(l) Li, Y.; Pang, H.; Wu, D.; Li, Z.; Wang, W.; Wei, H.; Fu, Y.; Yin, G. Angew. Chem.. Int. Ed. 2019, 58, 8872.
doi: 10.1002/anie.v58.26 pmid: 31386387 |
|
|
(m) Gao, T. T.; Zhang, W. W.; Sun, X.; Lu, H. X.; Li, B. J. J. Am. Chem. Soc. 2019, 141, 4670.
doi: 10.1021/jacs.8b13520 pmid: 31386387 |
|
|
(n) Kaiser, D.; Noble, A.; Fasano, V.; Aggarwal, V. K. J. Am. Chem. Soc. 2019, 141, 14104.
doi: 10.1021/jacs.9b07564 pmid: 31386387 |
|
|
(o) Liu, X.; Ming, W.; Friedrich, A.; Kerner, F.; Marder, T. B. Angew. Chem.. Int. Ed. 2020, 59, 304.
doi: 10.1002/anie.v59.1 pmid: 31386387 |
|
| [2] |
(a) Endo, K.; Ohkubo, T.; Hirokami, M.; Shibata, T. J. Am. Chem. Soc. 2010, 132, 11033.
doi: 10.1021/ja105176v pmid: 29379940 |
|
(b) Lee, J. C.; McDonald, R.; Hall, D. G. Nat. Chem. 2011, 3, 894.
doi: 10.1038/nchem.1150 pmid: 29379940 |
|
|
(c) Takaya, J.; Iwasawa, N. ACS Catal. 2012, 2, 1993.
doi: 10.1021/cs300320u pmid: 29379940 |
|
|
(d) Feng, X.; Jeon, H.; Yun, J. Angew. Chem.. Int. Ed. 2013, 52, 3989.
doi: 10.1002/anie.v52.14 pmid: 29379940 |
|
|
(e) Potter, B.; Szymaniak, A. A.; Edelstein, E. K.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 17918.
doi: 10.1021/ja510266x pmid: 29379940 |
|
|
(f) Coombs, J. R.; Zhang, L.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 16140.
doi: 10.1021/ja510081r pmid: 29379940 |
|
|
(g) Neeve, E. C.; Geier, S. J.; Mkhalid, I. A.; Westcott, S. A.; Marder, T. B. Chem. Rev. 2016, 116, 9091.
doi: 10.1021/acs.chemrev.6b00193 pmid: 29379940 |
|
|
(h) Kim, J.; Park, S.; Park, J.; Cho, S. H. Angew. Chem.. Int. Ed. 2016, 55, 1498.
doi: 10.1002/anie.v55.4 pmid: 29379940 |
|
|
(i) Murray, S. A.; Green, J. C.; Tailor, S. B.; Meek, S. J. Angew. Chem.. Int. Ed. 2016, 55, 9065.
doi: 10.1002/anie.v55.31 pmid: 29379940 |
|
|
(j) Zhao, H.; Tong, M.; Wang, H.; Xu, S. Org. Biomol. Chem. 2017, 15, 3418.
doi: 10.1039/C7OB00654C pmid: 29379940 |
|
|
(k) Miralles, N.; Maza, R. J.; Fernández, E. Adv. Synth. Catal. 2018, 360, 1306.
doi: 10.1002/adsc.v360.7 pmid: 29379940 |
|
|
(l) Nallagonda, R.; Padala, K.; Masarwa, A. Org. Biomol. Chem. 2018, 16, 1050.
doi: 10.1039/c7ob02978k pmid: 29379940 |
|
|
(m) Wu, C. Q.; Wang, J. B. Tetrahedron Lett. 2018, 59, 2128.
doi: 10.1016/j.tetlet.2018.04.061 pmid: 29379940 |
|
|
(n) Sun, W.; Wang, L.; Xia, C.; Liu, C. Angew. Chem.. Int. Ed. 2018, 57, 5501.
doi: 10.1002/anie.v57.19 pmid: 29379940 |
|
| [3] |
(a) Matteson, D. S.; Moody, R. J. J. Am. Chem. Soc. 1977, 99, 3196.
doi: 10.1021/ja00451a071 pmid: 25799147 |
|
(b) Coombs, J. R.; Zhang, L.; Morken, J. P. Org. Lett. 2015, 17, 1708.
doi: 10.1021/acs.orglett.5b00480 pmid: 25799147 |
|
| [4] |
(a) Hong, K.; Liu, X.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 10581.
doi: 10.1021/ja505455z pmid: 25019925 |
|
(b) Jo, W.; Kim, J.; Choi, S.; Cho, S. H. Angew. Chem.. Int. Ed. 2016, 55, 9690.
doi: 10.1002/anie.v55.33 pmid: 25019925 |
|
|
(c) Liu, X.; Deaton, T. M.; Haeffner, F.; Morken, J. P. Angew. Chem.. Int. Ed. 2017, 56, 11485.
doi: 10.1002/anie.v56.38 pmid: 25019925 |
|
| [5] |
(a) Endo, K.; Ohkubo, T.; Shibata, T. Org. Lett. 2011, 13, 3368.
doi: 10.1021/ol201115k |
|
(b) Endo, K.; Ohkubo, T.; Ishioka, T.; Shibata, T. J. Org. Chem. 2012, 77, 4826.
doi: 10.1021/jo3004293 |
|
|
(c) Zhang, Z. Q.; Yang, C. T.; Liang, L. J.; Xiao, B.; Lu, X.; Liu, J. H.; Sun, Y. Y.; Marder, T. B.; Fu, Y. Org. Lett. 2014, 16, 6342.
doi: 10.1021/ol503111h |
|
|
(d) Li, F.; Zhang, Z. Q.; Lu, X.; Xiao, B.; Fu, Y. Chem. Commun. 2017, 53, 3551.
doi: 10.1039/C7CC00129K |
|
| [6] |
(a) Li, H.; Zhang, Z.; Shangguan, X.; Huang, S.; Chen, J.; Zhang, Y.; Wang, J. Angew. Chem.. Int. Ed. 2014, 53, 11921.
doi: 10.1002/anie.v53.44 |
|
(b) Potter, B.; Szymaniak, A. A.; Edelstein, E. K.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 17918.
doi: 10.1021/ja510266x |
|
| [7] |
(a) Ebrahim-Alkhalil, A.; Zhang, Z. Q.; Gong, T. J.; Su, W.; Lu, X. Y.; Xiao, B.; Fu, Y. Chem. Commun. 2016, 52, 4891.
doi: 10.1039/C5CC09817C |
|
(b) Murray, S. A.; Liang, M. Z.; Meek, S. J. J. Am. Chem. Soc. 2017, 139, 14061.
doi: 10.1021/jacs.7b09309 |
|
| [8] |
(a) Joannou, M. V.; Moyer, B. S.; Goldfogel, M. J.; Meek, S. J. Angew. Chem.. Int. Ed. 2015, 54, 14141.
doi: 10.1002/anie.v54.47 |
|
(b) Joannou, M. V.; Moyer, B. S.; Meek, S. J. J. Am. Chem. Soc. 2015, 137, 6176.
doi: 10.1021/jacs.5b03477 |
|
|
(c) Park, J.; Lee, Y.; Kim, J.; Cho, S. H. Org. Lett. 2016, 18, 1210.
doi: 10.1021/acs.orglett.6b00376 |
|
|
(d) Kim, J.; Ko, K.; Cho, S. H. Angew. Chem.. Int. Ed. 2017, 56, 11584.
doi: 10.1002/anie.v56.38 |
|
| [9] |
(a) Shi, Y.; Hoveyda, A. H. Angew. Chem.. Int. Ed. 2016, 55, 3455.
doi: 10.1002/anie.v55.10 |
|
(b) Liu, X.; Sun, C.; Mlynarski, S.; Morken, J. P. Org. Lett. 2018, 20, 1898.
doi: 10.1021/acs.orglett.8b00439 |
|
| [10] |
Matteson, D. S.; Moody, R. J. Organometallic. 1982, 1, 20.
doi: 10.1021/om00061a005 |
| [11] |
(a) Hata, T.; Kitagawa, H.; Masai, H.; Kurahashi, T.; Shimizu, M.; Hiyama, T. Angew. Chem.. Int. Ed. 2001, 40, 790.
doi: 10.1002/1521-3773(20010216)40:4【-逻*辑*与-】amp;lt;【-逻*辑*与-】amp;gt;1.0.CO;2-X |
|
(b) Kurahashi, T.; Hata, T.; Masai, H.; Kitagawa, H.; Shimizu, M.; Hiyama, T. Tetrahedro. 2002, 58, 6381.
doi: 10.1016/S0040-4020(02)00648-8 |
|
|
(c) Shimizu, M.; Schelper, M.; Nagao, I.; Shimono, K.; Kurahashi, T.; Hiyama, T. Chem. Lett. 2006, 35, 1222.
doi: 10.1246/cl.2006.1222 |
|
| [12] |
(a) Endo, K.; Hirokami, M.; Shibata, T. Synlet. 2009, 1331.
|
|
(b) Barbeyron, R.; Benedetti, E.; Cossy, J.; Vasseur, J. J.; Arseniyadis, S.; Smietana, M. Tetrahedro. 2014, 70, 8431.
doi: 10.1016/j.tet.2014.06.026 |
|
| [13] |
(a) Cho, S. H.; Hartwig, J. F. Chem. Sci. 2014, 5, 694.
doi: 10.1039/C3SC52824C pmid: 31386387 |
|
(b) Palmer, W. N.; Obligacion, J. V.; Pappas, I.; Chirik, P. J. J. Am. Chem. Soc. 2016, 138, 766.
doi: 10.1021/jacs.5b12249 pmid: 31386387 |
|
|
(c) Yamamoto, T.; Ishibashi, A.; Suginome, M. Org. Lett. 2019, 21, 6235.
doi: 10.1021/acs.orglett.9b02112 pmid: 31386387 |
|
| [14] |
(a) Wang, L.; Zhang, T.; Sun, W.; He, Z.; Xia, C.; Lan, Y.; Liu, C. J. Am. Chem. Soc. 2017, 139, 5257.
doi: 10.1021/jacs.7b02518 pmid: 28306251 |
|
(b) Li, J.; Wang, H.; Qiu, Z.; Huang, C.-Y.; Li, C.-J. J. Am. Chem. Soc. 2020, 142, 13011.
doi: 10.1021/jacs.0c03813 pmid: 28306251 |
|
| [15] |
Wang, B.; Zhang, X.; Cao, Y.; Zou, L.; Qi, X.; Lu, Q. Angew. Chem.. Int. Ed. 2023, 62, e202218179.
|
| [16] |
(a) Westcott, S. A.; Blom, H. P.; Marder, T. B.; Baker, R. T. J. Am. Chem. Soc. 1992, 114, 8863.
doi: 10.1021/ja00049a019 |
|
(b) Burgess, K.; Van der Donk, W. A.; Westcott, S. A.; Marder, T. B.; Baker, R. T.; Calabrese, J. C. J. Am. Chem. Soc. 1992, 114, 9350.
doi: 10.1021/ja00050a015 |
|
|
(c) Westcott, S. A.; Marder, T. B.; Baker, R. T. Organometallic. 1993, 12, 975.
doi: 10.1021/om00027a058 |
|
|
(d) Garon, C. N.; McIsaac, D. I.; Vogels, C. M.; Decken, A.; Williams, I. D.; Kleeberg, C.; Marder, T. B.; Westcott, S. A. Dalton Trans. 2009, 1624.
|
|
|
(e) Wu, J. Y.; Moreau, B.; Ritter, T. J. Am. Chem. Soc. 2009, 131, 12915.
doi: 10.1021/ja9048493 |
|
|
(f) Tseng, K.-N. T.; Kampf, J. W.; Szymczak, N. K. ACS Catal. 2014, 5, 411.
doi: 10.1021/cs501820w |
|
|
(g) Wang, G.; Liang, X.; Chen, L.; Gao, Q.; Wang, J. G.; Zhang, P.; Peng, Q.; Xu, S. Angew. Chem.. Int. Ed. 2019, 58, 8187.
doi: 10.1002/anie.v58.24 |
|
|
(h) Chen, X.; Cheng, Z. Y.; Lu, Z. ACS Catal. 2019, 9, 4025.
doi: 10.1021/acscatal.8b05135 |
|
| [17] |
Li, L.; Gong, T.; Lu, X.; Xiao, B.; Fu, Y. Nat. Commun. 2017, 8, 345.
doi: 10.1038/s41467-017-00363-4 |
| [18] |
(a) Teo, W. J.; Ge, S. Angew. Chem.. Int. Ed. 2018, 57, 12935.
doi: 10.1002/anie.v57.39 |
|
(b) Teo, W. J.; Ge, S. Angew. Chem. Int. Ed. 2018, 57, 1654.
doi: 10.1002/anie.v57.6 |
|
| [19] |
Wang, X.; Cui, X.; Li, S.; Wang, Y.; Xia, C.; Jiao, H.; Wu, L. Angew. Chem.. Int. Ed. 2020, 59, 13608.
doi: 10.1002/anie.v59.32 |
| [20] |
Manßen, M.; Schafer, L. L. Chem. Soc. Rev. 2020, 49, 6947.
doi: 10.1039/D0CS00229A |
| [21] |
(a) Uwe Rosenthal V. V. B. In Titanium and Zirconium in Organic Synthesis, Wiley-VCH, Weinheim, 2002, pp. 355-389.
|
|
(b) Oakes, D. C. H.; Gibson, V. C.; White, A. J. P.; Williams, D. J. Inorg. Chem. 2006, 45, 3476.
doi: 10.1021/ic060146k |
|
|
(c) Hayatifar, M.; Pampaloni, G.; Bernazzani, L.; Capacchione, C.; Kissin, Y. V.; Raspolli Galletti, A. M. Polym. Int. 2014, 63, 560.
doi: 10.1002/pi.4558 |
|
|
(d) Ma, Y.; Lobkovsky, E. B.; Coates, G. W. Dalton Trans. 2015, 44, 12265.
doi: 10.1039/C5DT01104C |
|
|
(e) Ryken, S. A.; Schafer, L. L. Acc. Chem. Res. 2015, 48, 2576.
doi: 10.1021/acs.accounts.5b00224 |
|
| [22] |
(a) Bareille, L.; Becht, S.; Cui, J. L.; Le Gendre, P.; Moïse, C. Organometallic. 2005, 24, 5802.
doi: 10.1021/om050533m |
|
(b) Garcia, J.; Meyer, D. J. M.; Guillaneux, D.; Moreau, J. J. E.; Wong Chi Man, M. J. Organomet. Chem. 2009, 694, 2427.
doi: 10.1016/j.jorganchem.2009.03.018 |
|
| [23] |
(a) Cao, C.; Ciszewski, J. T.; Odom, A. L. Organometallic. 2001, 20, 5011.
doi: 10.1021/om010595m |
|
(b) Heutling, A.; Pohlki, F.; Bytschkov, I.; Doye, S. Angew. Chem.. Int. Ed. 2005, 44, 2951.
doi: 10.1002/anie.v44:19 |
|
|
(c) Müller, T. E.; Hultzsch, K. C.; Yus, M.; Foubelo, F.; Tada, M. Chem. Rev. 2008, 108, 3795.
doi: 10.1021/cr0306788 |
|
| [24] |
Perrier, A.; Comte, V.; Moïse, C.; Le Gendre, P. Chem.-Eur. J. 2010, 16, 64.
doi: 10.1002/chem.200901863 pmid: 19918817 |
| [25] |
(a) Burgess, K.; van der Donk, W. A. Tetrahedron Lett. 1993, 34, 6817.
doi: 10.1016/S0040-4039(00)91803-3 |
|
(b) He, X.; Hartwig, J. F. J. Am. Chem. Soc. 1996, 118, 1696.
doi: 10.1021/ja9516773 |
|
|
(c) Hartwig, J. F.; Muhoro, C. N. Organometallic. 2000, 19, 30.
doi: 10.1021/om990507m |
|
| [26] |
Bajgur, C. S.; Tikkanen, W.; Petersen, J. L. Inorg. Chem. 1985, 24, 2539.
doi: 10.1021/ic00210a015 |
| [27] |
Molander, G. A.; Brown, A. R. J. Org. Chem. 2006, 71, 9681.
pmid: 17168585 |
| [28] |
Liu, H.; Guo, Q.; Chen, C.; Wang, M.Xu. Z. Org. Chem. Front. 2018, 5, 1522.
doi: 10.1039/C8QO00120K |
| [1] | Yang Li, Yanan Dong, Yuehui Li. Efficient Synthesis of Nitrile Compounds through Amide Conversion via N-Boroamide Intermediates [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 638-643. |
| [2] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [3] | Jiyu Liu, Shengyu Li, Kuan Chen, Yin Zhu, Yuan Zhang. Triphenylamine-Based Ordered Mesoporous Polymer as a Metal-Free Photocatalyst for Oxidation of Thiols to Disulfide [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 605-612. |
| [4] | Shuang Yang, Xinqiang Fang. Kinetic Resolutions Enabled by N-Heterocyclic Carbene Catalysis: An Update [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 448-480. |
| [5] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [6] | Wanting Chen, Xiongwei Zhong, Jiale Xing, Changshu Wu, Yang Gao. Progress in Asymmetric Catalytic Synthesis of C—N Axis Chiral Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 349-377. |
| [7] | Jing Huang, Yihua Yang, Zhanhui Zhang, Shouxin Liu. Recent Progress on Green Methods and Technologies for Efficient Formation of Amide Bonds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 409-420. |
| [8] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [9] | Yanshuo Zhu, Hongyan Wang, Penghua Shu, Ke'na Zhang, Qilin Wang. Recent Advances on Alkoxy Radicals-Mediated C(sp3)—H Bond Functionalization via 1,5-Hydrogen Atom Transfer [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 1-17. |
| [10] | Hongqiong Zhao, Miao Yu, Dongxue Song, Qi Jia, Yingjie Liu, Yubin Ji, Ying Xu. Progress on Decarboxylation and Hydroxylation of Carboxylic Acids [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 70-84. |
| [11] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [12] | Xianqiang Meng, Yi Yang, Wanjie Liang, Jingtao Wang, Rongkui Zhang, Hui Liu. Palladium-Catalyzed Regioselective Aryl Phenoxylation of Allenamide [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 224-231. |
| [13] | Mengzhu Li, Boying Meng, Wenjie Lan, Bin Fu. Synthesis of 2,3-Disubstituted Dihydrobenzofurans from o-Quinone Methides and Sulfur Ylides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 195-203. |
| [14] | Hong'en Tong, Hongyu Guo, Rong Zhou. Progress on Visible-Light Promoted Addition Reactions of Inert C—H Bonds to Carbonyls [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 54-69. |
| [15] | Quanbin Jiang. Progress in Synthesis of Axially Chiral Compounds through aza-Vinylidene o-Quinone Methide Intermediates [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 159-172. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||