有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1288-1318.DOI: 10.6023/cjoc202009025 上一篇 下一篇
所属专题: 热点论文虚拟合集
综述与进展
收稿日期:2020-09-09
修回日期:2020-10-21
发布日期:2020-11-19
通讯作者:
王晓娜
基金资助:
Xinyue Zhou1, Zongxian Liang1, Xiao-Na Wang1,*( )
)
Received:2020-09-09
Revised:2020-10-21
Published:2020-11-19
Contact:
Xiao-Na Wang
About author:Supported by:文章分享
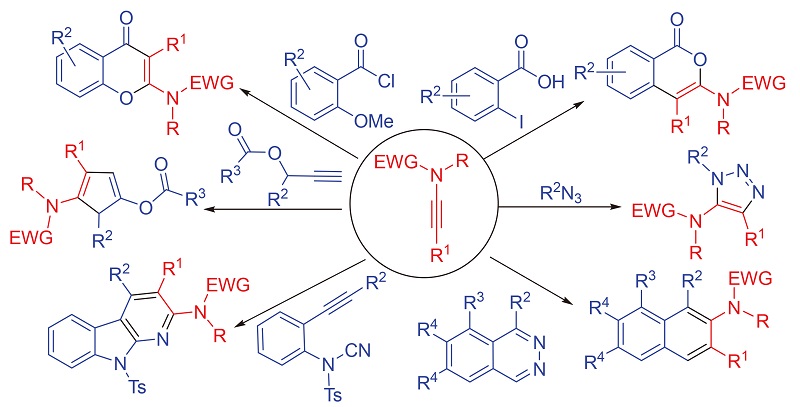
炔酰胺作为一种杂原子取代的炔, 具有独特的结构特征, 其反应活性和稳定性能够达到很好的平衡, 已作为一种多功能型的有机合成子被广泛应用于有机化学中. 尤其是随着炔酰胺高效型和原子经济型制备方法的出现, 炔酰胺参与的反应类型渐渐趋于多样化. 在这多样化的反应类型中有关炔酰胺成环反应的研究占主要部分, 这跟炔酰胺的结构特征密切相关, 炔酰胺炔基上的α碳具有一定的亲电性, β碳具有一定的亲核性, 有利于成环反应的发生. 另一方面, 炔酰胺参与成环反应所生成的含氮环状化合物, 为大量活性天然产物和药物分子的构建提供了重要的结构单元, 因此, 关于炔酰胺参与的成环反应的研究具有重要的意义. 概述了近年来炔酰胺参与成环反应的最新研究进展.
周欣悦, 梁宗显, 王晓娜. 近年来炔酰胺参与的成环反应研究进展[J]. 有机化学, 2021, 41(4): 1288-1318.
Xinyue Zhou, Zongxian Liang, Xiao-Na Wang. Recent Advances in the Ring-Forming Reactions of Ynamides[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1288-1318.


| [1] |
Viehe, H.G. Angew. Chem., Int. Ed. 1963, 2,477.
|
| [2] |
Janousek, Z.; Collard, J.; Viehe, H.G. Angew. Chem., Int. Ed. 1972, 11,917.
doi: 10.1002/(ISSN)1521-3773 |
| [3] |
(a) Evano, G.; Coste, A.; Jouvin, K. Angew. Chem., Int. Ed. 2010, 49,2840.
doi: 10.1002/anie.200905817 pmid: 32869969 |
|
(b) DeKorver, K.A.; Li, H.; Lohse, A.G.; Hayashi, R.; Lu, Z.; Zhang, Y.; Hsung, R.P. Chem. Rev. 2010, 110,5064.
doi: 10.1021/cr100003s pmid: 32869969 |
|
|
(c) Wang, X.-N.; Yeom, H.-S.; Fang, L.-C.; He, S.; Ma, Z.-X.; Kedrowski, B.L.; Hsung, R.P. Acc. Chem. Res. 2014, 47,560.
doi: 10.1021/ar400193g pmid: 32869969 |
|
|
(d) Evano, G.; Theunissen, C.; Lecomte, M. Aldrichim. Acta 2015, 48,59.
pmid: 32869969 |
|
|
(e) Pan, F.; Shu, C.; Ye, L.-W. Org. Biomol. Chem. 2016, 14,9456.
doi: 10.1039/c6ob01774f pmid: 32869969 |
|
|
(f) Liao, Y.; Zhu, L.; Yu, Y.; Chen, G.; Huang, X. Chin. J. Org. Chem. 2017, 37,2785. (in Chinese)
doi: 10.6023/cjoc201708021 pmid: 32869969 |
|
|
( 廖云, 朱磊, 俞颖华, 陈贵, 黄学良, 有机化学, 2017, 37,2785.) 2578dc51-76c9-4272-b262-bd95d94f25c8
doi: 10.6023/cjoc201708021 pmid: 32869969 |
|
|
(g) Zhou, B.; Tan, T.-D.; Zhu, X.-Q.; Shang, M.; Ye, L.-W. ACS Catal. 2019, 9,6393.
doi: 10.1021/acscatal.9b01851 pmid: 32869969 |
|
|
(h) Hong, F.-L.; Ye, L.-W. Acc. Chem. Res. 2020, 53,2003.
doi: 10.1021/acs.accounts.0c00417 pmid: 32869969 |
|
| [4] |
Balieu, S.; Toutah, K.; Carro, L.; Chamoreau, L.-M.; Rousselière, H.; Courillon, C. Tetrahedron Lett. 2011, 52,2876. fd0395e4-17bb-4cea-b580-bf27d1e41cf9
doi: 10.1016/j.tetlet.2011.03.118 |
| [5] |
Marion, F.; Courillon, C.; Malacria, M. Org. Lett. 2003, 5,5095.
doi: 10.1021/ol036177q pmid: 14682773 |
| [6] |
(a) Chemla, F.; Dulong, F.; Ferreira, F.; Nüllen, M.; Pérez-Luna, A. Synthesis 2011,1347.
pmid: 30495946 |
|
(b) Romain, E.; Fopp, C.; Chemla, F.; Ferreira, F.; Jackowski, O.; Oestreich, M.; Perez-Luna, A. Angew. Chem., Int. Ed. 2014, 53,11333.
doi: 10.1002/anie.201407002 pmid: 30495946 |
|
|
(c) de la Vega-Hernández, K.; Romain, E.; Coffinet, A.; Bijouard, K.; Gontard, G.; Chemla, F.; Ferreira, F.; Jackowski, O.; Perez-Luna, A. J. Am. Chem. Soc. 2018, 140,17632.
pmid: 30495946 |
|
| [7] |
Dutta, S.; Mallick, R.K.; Prasad, R.; Gandon, V.; Sahoo, A.K. Angew. Chem., Int. Ed. 2019, 58,2289.
|
| [8] |
Casse, M.; Nisole, C.; Dossmann, H.; Gimbert, Y.; Fourquez, J.-M.; Haberkorn, L.; Ollivier, C.; Fensterbank, L. Sci. China: Chem. 2019, 62,1542.
|
| [9] |
Wakamatsu, H.; Sakagami, M.; Hanata, M.; Takeshita, M.; Mori, M. Macromol. Symp. 2010, 293,5.
|
| [10] |
Wakamatsu, H.; Sasaki, Y.; Kawahata, M.; Yamaguchi, K.; Yoshimura, Y. Synthesis 2018, 50,3467.
|
| [11] |
Poloukhtine, A.; Rassadin, V.; Kuzmin, A.; Popik, V.V. J. Org. Chem. 2010, 75,5953.
pmid: 20684502 |
| [12] |
Gati, W.; Rammah, M.M.; Rammah, M.B.; Couty, F.; Evano, G. J. Am. Chem. Soc. 2012, 134,9078.
pmid: 22583001 |
| [13] |
Meng, T.-J.; Chen, R.-X.; Liu, L.-T.; Wang, T.; Liu, X.-M.; Zhao, W.-X. Chin. J. Org. Chem. 2015, 35,2108. (in Chinese)
|
|
( 孟团结, 陈荣祥, 刘澜涛, 王涛, 刘新明, 赵文献, 有机化学, 2015, 35,2108.)
|
|
| [14] |
Kong, Y.; Jiang, K.; Cao, J.; Fu, L.; Yu, L.; Lai, G.; Cui, Y.; Hu, Z.; Wang, G. Org. Lett. 2013, 15,422.
pmid: 23301862 |
| [15] |
Willumstad, T.P.; Boudreau, P.D.; Danheiser, R.L. J. Org. Chem. 2015, 80,11794.
|
| [16] |
Liu, H.; Yang, Y.; Wang, S.; Wu, J.; Wang, X.-N.; Chang, J. Org. Lett. 2015, 17,4472.
pmid: 26332185 |
| [17] |
Lecomte, M.; Evano, G. Angew. Chem., Int. Ed. 2016, 55,4547.
|
| [18] |
Wang, Y.; Lin, J.; Wang, X; Wang, G.; Zhang, X.; Yao, B.; Zhao, Y.; Yu, P.; Lin, B.; Liu, Y.; Cheng, M. Chem.-Eur. J. 2018, 24,4026.
pmid: 29168592 |
| [19] |
Brutiu, B.R.; Bubeneck, W.A.; Cvetkovic, O.; Li, J.; Maulide, N. Monatsh. Chem. 2018, 150,3.
pmid: 30662090 |
| [20] |
Zhang, J.; Li, S.; Qiao, Y.; Peng, C.; Wang, X.-N.; Chang, J. Chem. Commun. 2018, 54,12455.
|
| [21] |
Yoo, H.J.; Youn, S.W. Org. Lett. 2019, 21,3422.
pmid: 31012589 |
| [22] |
Li, L.; Zhou, B.; Wang, Y.-H.; Shu, C.; Pan, Y.-F.; Lu, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2015, 54,8245.
|
| [23] |
Li, H.-H.; Ye, S.-H.; Chen, Y.-B.; Luo, W.-F.; Qian, P.-C.; Ye, L.-W. Chin. J. Chem. 2020, 38,263.
|
| [24] |
Nishimura, T.; Takiguchi, Y.; Maeda, Y.; Hayashi, T. Adv. Synth. Catal. 2013, 355,1374.
|
| [25] |
Liao, Y.; Lu, Q.; Chen, G.; Yu, Y.; Li, C.; Huang, X. ACS Catal. 2017, 7,7529.
|
| [26] |
Okamoto, N.; Yanada, R.; Sueda, T. Eur. J. Org. Chem. 2019,691.
|
| [27] |
Greenaway, R.L.; Campbell, C.D.; Holton, O.T.; Russell, C.A.; Anderson, E.A. Chem.-Eur. J. 2011, 17,14366.
|
| [28] |
Greenaway, R.L.; Campbell, C.D.; Chapman, H.A.; Anderson, E.A. Adv. Synth. Catal. 2012, 354,3187.
|
| [29] |
Cao, J.; Xu, Y.; Kong, Y.; Cui, Y.; Hu, Z.; Wang, G.; Deng, Y.; Lai, G. Org. Lett. 2012, 14,38.
pmid: 22126429 |
| [30] |
Huang, H.; He, G.; Zhu, G.; Zhu, X.; Qiu, S.; Zhu, H. J. Org. Chem. 2015, 80,3480.
|
| [31] |
Liu, H.; Yang, Y.; Wu, J.; Wang, X.-N.; Chang, J. Chem. Commun. 2016, 52,6801.
|
| [32] |
Reddy, A.S.; Kumari, A.L. S.; Swamy, K.C. K. Tetrahedron 2017, 73,2766.
|
| [33] |
Bhunia, S.; Chang, C.-J.; Liu, R.-S. Org. Lett. 2012, 14,5522.
|
| [34] |
Shen, W.-B.; Xiao, X.-Y.; Sun, Q.; Zhou, B.; Zhu, X.-Q.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56,605.
|
| [35] |
Hashmi, A.S. K.; Schuster, A.M.; Zimmer, M.; Rominger, F. Chem.-Eur. J. 2011, 17,5511.
pmid: 21491523 |
| [36] |
Gati, W.; Couty, F.; Boubaker, T.; Rammah, M.M.; Rammah, M.B.; Evano, G. Org. Lett. 2013, 15,3122.
|
| [37] |
Reddy, A.S.; Reddy, M.N.; Swamy, K.C. K. RSC Adv. 2014, 4,28359.
|
| [38] |
Nickel, J.; Fernández, M.; Klier, L.; Knochel, P. Chem.-Eur. J. 2016, 22,14397.
|
| [39] |
Baguia, H.; Deldaele, C.; Romero, E.; Michelet, B.; Evano, G. Synthesis 2018, 50,3022.
|
| [40] |
Hong, F.-L.; Wang, Z.-S.; Wei, D.-D.; Zhai, T.-Y.; Deng, G.-C.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2019, 141,16961.
pmid: 31557018 |
| [41] |
Hong, F.-L.; Chen, Y.-B.; Ye, S.-H.; Zhu, G.-Y.; Zhu, X.-Q.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142,7618.
pmid: 32237743 |
| [42] |
Liu, X.; Wang, Z.-S.; Zhai, T.-Y.; Luo, C.; Zhang, Y.-P.; Chen, Y.-B.; Deng, C.; Liu, R.-S.; Ye, L.-W. Angew. Chem., Int. Ed. 2020, 59,17984.
|
| [43] |
Garcia, P.; Harrak, Y.; Diab, L.; Cordier, P.; Ollivier, C.; Gandon, V.; Malacria, M.; Fensterbank, L.; Aubert, C. Org. Lett. 2011, 13,2952.
pmid: 21534621 |
| [44] |
Liang, G.; Ji, Y.; Liu, H.; Pang, Y.; Zhou, B.; Cheng, M.; Liu, Y.; Lin, B.; Liu, Y. Adv. Synth. Catal. 2020, 362,192.
|
| [45] |
Ieawsuwan, W.; Ruchirawat, S. Heterocycles 2019, 99,100.
|
| [46] |
Blanco Jaimes, M.C.; Weingand, V.; Rominger, F.; Hashmi, A.S. K. Chem.-Eur. J. 2013, 19,12504.
|
| [47] |
Rettenmeier, E.; Schuster, A.M.; Rudolph, M.; Rominger, F.; Gade, C.A.; Hashmi, A.S. K. Angew. Chem., Int. Ed. 2013, 52,5880.
|
| [48] |
Tokimizu, Y.; Oishi, S.; Fujii, N.; Ohno, H. Org. Lett. 2014, 16,3138.
|
| [49] |
Tokimizu, Y.; Wieteck, M.; Rudolph, M.; Oishi, S.; Fujii, N.; Hashmi, A.S. K.; Ohno, H. Org. Lett. 2015, 17,604.
pmid: 25611870 |
| [50] |
Shu, C.; Wang, Y.-H.; Zhou, B.; Li, X.-L.; Ping, Y.-F.; Lü, X.; Ye, L.-W. J. Am. Chem. Soc. 2015, 137,9567.
|
| [51] |
Shu, C.; Wang, Y.-H.; Shen, C.-H.; Ruan, P.-P.; Lü, X.; Ye, L.-W. Org. Lett. 2016, 18,3254.
pmid: 27331406 |
| [52] |
Jin, H.; Tian, B.; Song, X.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S. K. Angew. Chem., Int. Ed. 2016, 55,12688.
|
| [53] |
Shen, W.-B.; Sun, Q.; Li, L.; Liu, X.; Zhou, B.; Yan, J.-Z.; Lü, X.; Ye, L.-W. Nat. Commun. 2017, 8,1748.
pmid: 29170497 |
| [54] |
Ito, M.; Kawasaki, R.; Kanyiva, K.S.; Shibata, T. Chem.-Eur. J. 2018, 24,3721.
doi: 10.1002/chem.201800314 pmid: 29372752 |
| [55] |
Xu, W.; Wang, G.; Xie, X.; Liu, Y. Org. Lett. 2018, 20,3273.
doi: 10.1021/acs.orglett.8b01145 pmid: 29767992 |
| [56] |
Hsu, Y.-C.; Hsieh, S.-A.; Liu, R.-S. Chem.-Eur. J. 2019, 25,5288.
|
| [57] |
Vanjari, R.; Dutta, S.; Gogoi, M.P.; Gandon, V.; Sahoo, A.K. Org. Lett. 2018, 20,8077.
pmid: 30540197 |
| [58] |
Rode, N.D.; Arcadi, A.; Nicola, A.D.; Marinelli, F.; Michelet, V. Org. Lett. 2018, 20,5103.
|
| [59] |
Febvay, J.; Sanogo, Y.; Retailleau, P.; Gogoi, M.P.; Sahoo, A.K.; Marinetti, A.; Voituriez, A. Org. Lett. 2019, 21,9281.
pmid: 31762272 |
| [60] |
Li, H.; Hsung, R.P.; Dekorver, K.A.; Wei, Y. Org. Lett. 2010, 12,3780.
|
| [61] |
Schotes, C.; Mezzetti, A. Angew. Chem., Int. Ed. 2011, 50,3072.
|
| [62] |
Smith, D.L.; Chidipudi, S.R.; Goundry, W.R.; Lam, H.W. Org. Lett. 2012, 14,4934.
pmid: 22954424 |
| [63] |
Yuan, Y.; Bai, L.; Nan, J.; Liu, J.; Luan, X. Org. Lett. 2014, 16,4316.
|
| [64] |
Wang, X.-N.; Ma, Z.-X.; Deng, J.; Hsung, R.P. Tetrahedron Lett. 2015, 56,3463.
pmid: 26028784 |
| [65] |
Chen, L.; Cao, J.; Xu, Z.; Zheng, Z.-J.; Cui, Y.-M.; Xu, L.-W. Chem. Commun. 2016, 52,9574.
|
| [66] |
Yang, Y.; Liu, H.; Peng, C.; Wu, J.; Zhang, J.; Qiao, Y.; Wang, X.-N.; Chang, J. Org. Lett. 2016, 18,5022.
pmid: 27653170 |
| [67] |
Peng, C.; Zhang, J.; Xue, J.; Li, S.; Wang, X.-N.; Chang, J. J. Org. Chem. 2018, 83,9256.
pmid: 29978700 |
| [68] |
Davies, P.W.; Cremonesi, A.; Dumitrescu, L. Angew. Chem., Int. Ed. 2011, 50,8931.
|
| [69] |
Mackay, W.D.; Fistikci, M.; Carris, R.M.; Johnson, J.S. Org. Lett. 2014, 16,1626.
pmid: 24606195 |
| [70] |
(a) Zhou, A.-H.; He, Q.; Shu, C.; Yu, Y.-F.; Liu, S.; Zhao, T.; Zhang, W.; Lü, X.; Ye, L.-W. Chem. Sci. 2015, 6,1265.
pmid: 29560212 |
|
(b) Li, X.-L.; Wang, J.-Q.; Li, L.; Yin, Y.-W.; Ye, L.-W. Acta Chim. Sinica 2016, 74,49. (in Chinese)
pmid: 29560212 |
|
|
( 李新玲, 王佳琪, 李龙, 尹应武, 叶龙武, 化学学报, 2016, 74,49.)
pmid: 29560212 |
|
| [71] |
Yu, Y.; Chen, G.; Zhu, L.; Liao, Y.; Wu, Y.; Huang, X. J. Org. Chem. 2016, 81,8142.
pmid: 27569125 |
| [72] |
Zhao, Y.; Hu, Y.; Wang, C.; Li, X.; Wan, B. J. Org. Chem. 2017, 82,3935.
doi: 10.1021/acs.joc.7b00076 pmid: 28276692 |
| [73] |
Zhao, Y.; Hu, Y.; Li, X.; Wan, B. Org. Biomol. Chem. 2017, 15,3413.
|
| [74] |
Tian, X.; Song, L.; Han, C.; Zhang, C.; Wu, Y.; Rudolph, M.; Rominger, F.; Hashmi, A.S. K. Org. Lett. 2019, 21,2937.
pmid: 30964689 |
| [75] |
Lin, P.-P.; Han, X.-L.; Ye, G.-H.; Li, J.-L.; Li, Q.; Wang, H. J. Org. Chem. 2019, 84,12966.
doi: 10.1021/acs.joc.9b01750 pmid: 31490696 |
| [76] |
Mak, X.Y.; Crombie, A.L.; Danheiser, R.L. J. Org. Chem. 2011, 76,1852.
doi: 10.1021/jo2000308 pmid: 21322545 |
| [77] |
Pawar, S.K.; Vasu, D.; Liu, R.-S. Adv. Synth. Catal. 2014, 356,2411.
|
| [78] |
Duret, G.; Quinlan, R.; Martin, R.E.; Bisseret, P.; Neuburger, M.; Gandon, V.; Blanchard, N. Org. Lett. 2016, 18,1610.
doi: 10.1021/acs.orglett.6b00464 pmid: 26998920 |
| [79] |
Xue, J.; Gao, E.; Wang, X.-N.; Chang, J. Org. Lett. 2018, 20,6055.
|
| [80] |
Zhao, X.; Song, X.; Jin, H.; Zeng, Z.; Wang, Q.; Rudolph, M.; Rominger, F.; Hashmi, A.S. K. Adv. Synth. Catal. 2018, 360,2720.
|
| [81] |
Wu, H.; Liu, Y.; He, M.-X.; Wen, H.; Cao, W.; Chen, P.; Tang, Y. Org. Biomol. Chem. 2019, 17,8408.
pmid: 31478045 |
| [82] |
Nissen, F.; Richard, V.; Alayrac, C.; Witulski, B. Chem. Commun. 2011, 47,6656.
|
| [83] |
Garcia, P.; Evanno, Y.; George, P.; Sevrin, M.; Ricci, G.; Malacria, M.; Aubert, C.; Gandon, V. Chem.-Eur. J. 2012, 18,4337.
pmid: 22383395 |
| [84] |
Karad, S.N.; Liu, R.-S. Angew. Chem., Int. Ed. 2014, 53,9072.
|
| [85] |
Liang, H.; Bi, S.; Liu, Y.; Tang, Y.-N.; Liu, C. Org. Biomol. Chem. 2016, 14,2637.
doi: 10.1039/c5ob02568k pmid: 26908288 |
| [86] |
Liu, D.; Nie, Q.; Cai, M. Tetrahedron 2018, 74,3020.
|
| [87] |
Chen, P.; Song, C.-X.; Wang, W.-S.; Yu, X.-L.; Tang, Y. RSC Adv. 2016, 6,80055.
|
| [88] |
Zhang, J.; Zhang, Q.; Xia, B.; Wu, J.; Wang, X.-N.; Chang, J. Org. Lett. 2016, 18,3390.
pmid: 27366955 |
| [89] |
Wen, H.; Cao, W.; Liu, Y.; Wang, L.; Chen, P.; Tang, Y. J. Org. Chem. 2018, 83,13308.
pmid: 30353730 |
| [90] |
Zhang, J.; Guo, M.; Chen, Y.; Zhang, S.; Wang, X.-N.; Chang, J. Org. Lett. 2019, 21,1331.
doi: 10.1021/acs.orglett.9b00021 pmid: 30735400 |
| [91] |
Dateer, R.B.; Pati, K.; Liu, R.-S. Chem. Commun. 2012, 48,7200.
|
| [92] |
Pawar, S.K.; Sahani, R.L.; Liu, R.-S. Chem.-Eur. J. 2015, 21,10843.
pmid: 26094616 |
| [93] |
Jadhav, P.D.; Lu, X.; Liu, R.-S. ACS Catal. 2018, 8,9697.
|
| [94] |
Han, X.-L.; Liu, X.-G.; Lin, E.; Chen, Y.; Chen, Z.; Wang, H.; Li, Q. Chem. Commun. 2018, 54,11562.
|
| [95] |
Jin, H.; Rudolph, M.; Rominger, F.; Hashmi, A.S. K. ACS Catal. 2019, 9,11663.
|
| [96] |
Dekorver, K.A.; Hsung, R.P.; Lohse, A.G.; Zhang, Y. Org. Lett. 2010, 12,1840.
|
| [97] |
Brioche, J.; Meyer, C.; Cossy, J. Org. Lett. 2013, 15,1626.
doi: 10.1021/ol400402n pmid: 23496162 |
| [98] |
Zhou, B.; Li, L.; Zhu, X.-Q.; Yan, J.-Z.; Guo, Y.-L.; Ye, L.-W. Angew. Chem., Int. Ed. 2017, 56,4015.
|
| [99] |
Baker, T.; Davies, P.W. Eur. J. Org. Chem. 2019,5201.
|
| [100] |
Wang, Z.-S.; Chen, Y.-B.; Zhang, H.-W.; Sun, Z.; Zhu, C.; Ye, L.-W. J. Am. Chem. Soc. 2020, 142,3636.
|
| [1] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [2] | 高宝昌, 石雨, 田媛, 张治国, 张婧如, 孙宇峰, 毛国梁, 戴凌燕. 4-甲基-2-氧代-6-芳氨基-二氢-吡喃-3-腈衍生物的合成[J]. 有机化学, 2024, 44(2): 644-649. |
| [3] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [4] | 李梦竹, 孟博莹, 兰文捷, 傅滨. 邻亚甲醌与硫叶立德反应合成2,3-二取代苯并二氢呋喃化合物[J]. 有机化学, 2024, 44(1): 195-203. |
| [5] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [6] | 马虎, 黄丹凤, 王克虎, 唐朵朵, 冯杨, 任园园, 王君娇, 胡雨来. 3-(三氟甲基)吡唑类化合物的合成[J]. 有机化学, 2023, 43(9): 3257-3267. |
| [7] | 陈祖良, 魏颖静, 张俊良. 供体-受体氮杂环丙烷碳-碳键断裂的环加成反应研究进展[J]. 有机化学, 2023, 43(9): 3078-3088. |
| [8] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [9] | 樊思捷, 董武恒, 梁彩云, 王贵超, 袁瑶, 尹作栋, 张兆国. 可见光诱导的自由基环化反应构建4-芳基-1,2-二氢萘类化合物[J]. 有机化学, 2023, 43(9): 3277-3286. |
| [10] | 王熠, 张键, 刘飏子, 罗晓燕, 邓卫平. 钯催化不对称[3+4]环加成构建吲哚并环庚烷[J]. 有机化学, 2023, 43(8): 2864-2877. |
| [11] | 冯莹珂, 王贺, 崔梦行, 孙然, 王欣, 陈阳, 李蕾. 可见光诱导的新型官能化芳基异腈化合物的二氟烷基化环化反应[J]. 有机化学, 2023, 43(8): 2913-2925. |
| [12] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [13] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [14] | 蔡荣斌, 李冰, 周琪, 朱隆懿, 罗军. 4,8,9,10-四官能化的2-氮杂金刚烷及其2-氮杂原金刚烷骨架异构体的合成[J]. 有机化学, 2023, 43(6): 2217-2225. |
| [15] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||