有机化学 ›› 2021, Vol. 41 ›› Issue (11): 4208-4239.DOI: 10.6023/cjoc202106021 上一篇 下一篇
所属专题: 镍催化有机反应虚拟合辑; 热点论文虚拟合集
综述与进展
吴良a, 魏瀚林a, 陈建中b,*( ), 张万斌a,b,*(
), 张万斌a,b,*( )
)
收稿日期:2021-06-10
修回日期:2021-06-22
发布日期:2021-07-19
通讯作者:
陈建中, 张万斌
基金资助:
Liang Wua, Hanlin Weia, Jianzhong Chenb( ), Wanbin Zhanga,b(
), Wanbin Zhanga,b( )
)
Received:2021-06-10
Revised:2021-06-22
Published:2021-07-19
Contact:
Jianzhong Chen, Wanbin Zhang
Supported by:文章分享

过渡金属催化构筑碳-碳键的交叉偶联反应由于其高效性、高选择性而得到了研究人员的广泛关注. 近年来, 含C(sp3)—O键的亲电试剂由于其商业易得或容易合成、反应选择性高、环境友好等优点而被用来替代有机卤化物, 应用到构建C(sp3)—C键的交叉偶联反应中. 一系列高效催化体系得以陆续报道, 其中镍催化剂由于其储量丰富、价格便宜以及独特的催化活性和选择性而被逐步应用到此类反应中, 并取得了显著的成果. 综述了镍催化醇衍生物参与的偶联反应的最新研究进展, 主要包括镍催化甲醇或伯醇衍生物参与的偶联反应, 镍催化仲醇衍生物参与的偶联反应, 镍催化叔醇衍生物参与的偶联反应, 以及镍催化缩醛和N,O-缩醛衍生物参与的偶联反应等.
吴良, 魏瀚林, 陈建中, 张万斌. 镍催化醇衍生物构筑碳-碳键的偶联反应研究进展[J]. 有机化学, 2021, 41(11): 4208-4239.
Liang Wu, Hanlin Wei, Jianzhong Chen, Wanbin Zhang. Development of Nickel-Catalyzed Cross-Coupling of Alcohol Derivatives to Construct Carbon-Carbon Bonds[J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4208-4239.
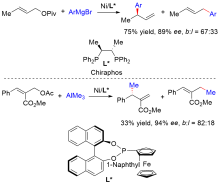

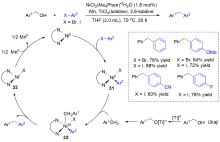
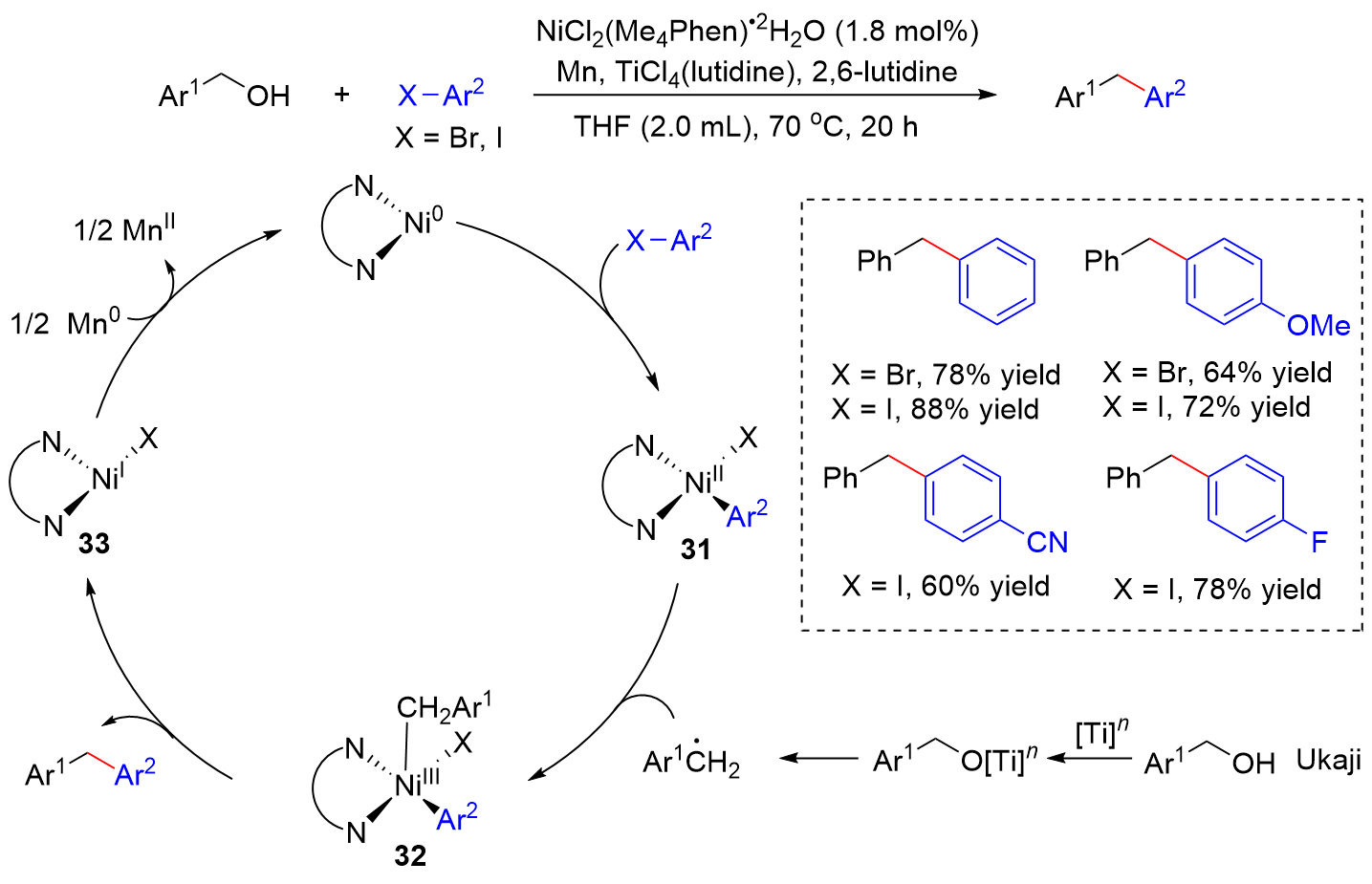


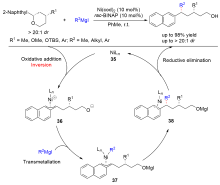

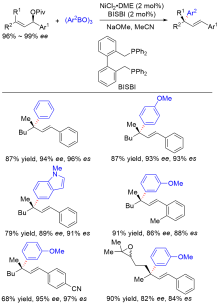
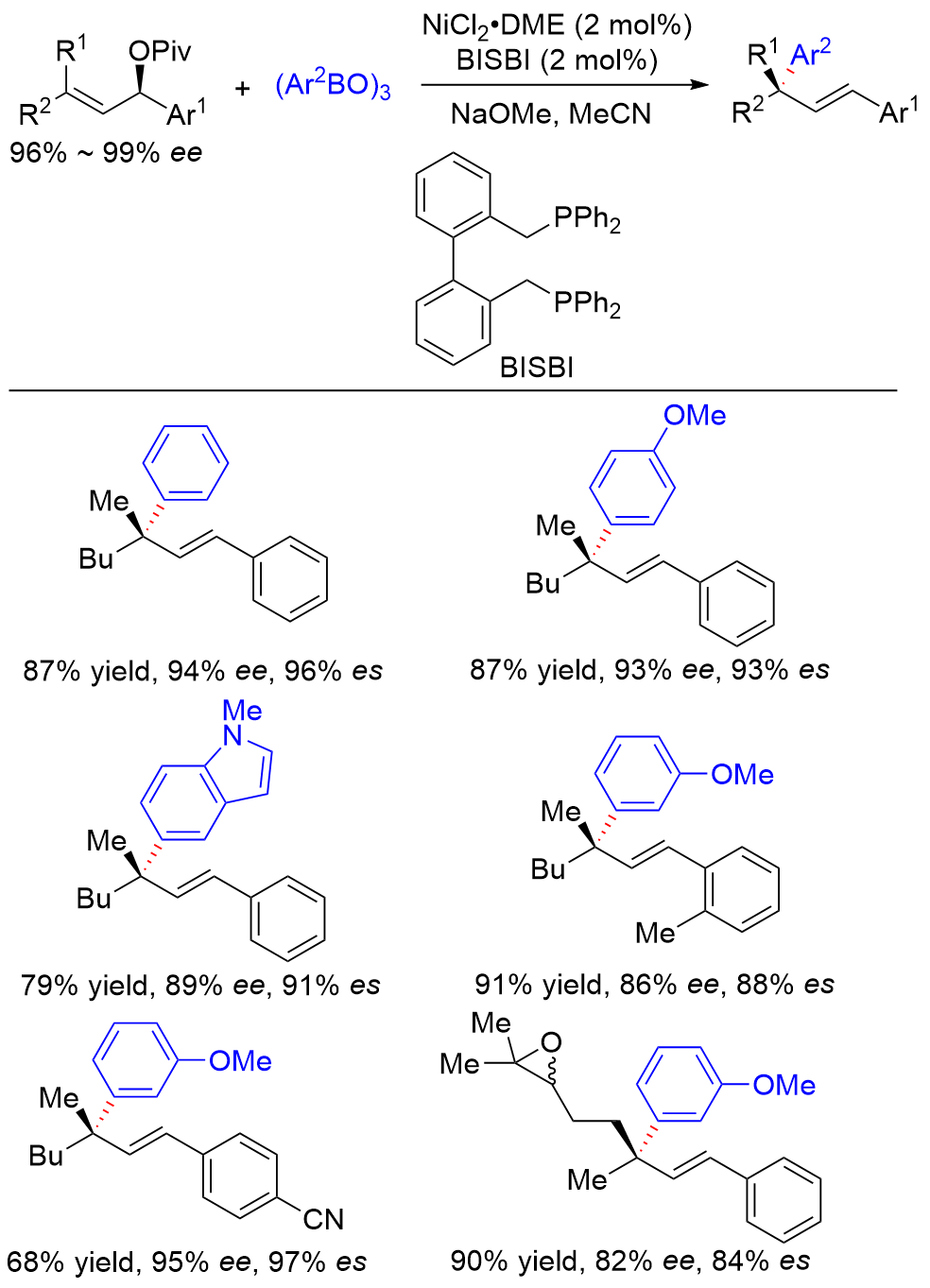
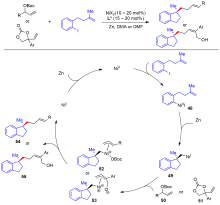
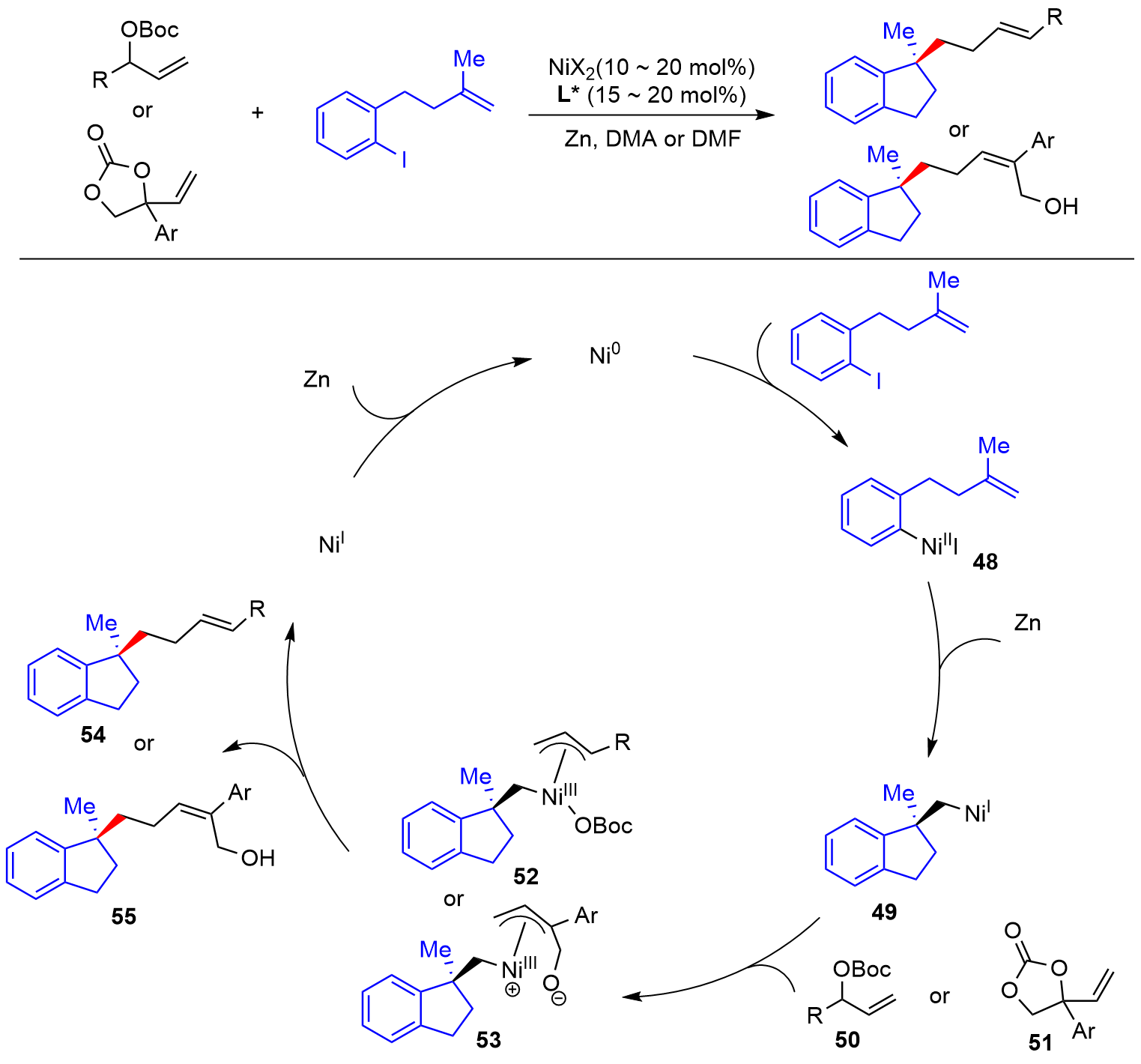
| [1] |
(a) Fagnou, K.; Lautens, M. Chem. Rev. 2003, 103, 169.
pmid: 26268813 |
|
(b) Cherney, A. H.; Kadunce, N. T.; Reisman, S. E. Chem. Rev. 2015, 115, 9587.
doi: 10.1021/acs.chemrev.5b00162 pmid: 26268813 |
|
| [2] |
(a) Wu, X. F.; Anbarasan, P.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 9047.
doi: 10.1002/anie.201006374 |
|
(b) Hanna, L. E.; Jarvo, E. R. Angew. Chem., Int. Ed. 2015, 54, 15618.
doi: 10.1002/anie.v54.52 |
|
|
(c) Li, Y.; Fan, Y.; Jia, Q. Chin. J. Org. Chem. 2019, 39, 350. (in Chinese)
doi: 10.6023/cjoc201806038 |
|
|
(李娅琼, 范玉航, 贾乾发, 有机化学, 2019, 39, 350.)
doi: 10.6023/cjoc201806038 |
|
| [3] |
(a) Knappke, C. E. I.; Grupe, S.; Gärtner, D.; Corpet, M.; Gosmini, C.; von Wangelin, A. J. Chem.-Eur. J. 2014, 20, 6828.
doi: 10.1002/chem.201402302 pmid: 24905555 |
|
(b) Moragas, T.; Correa, A.; Martin, R. Chem.-Eur. J. 2014, 20, 8242.
doi: 10.1002/chem.201402509 pmid: 24905555 |
|
|
(c) Weix, D. J. Acc. Chem. Res. 2015, 48, 1767.
doi: 10.1021/acs.accounts.5b00057 pmid: 24905555 |
|
|
(d) Gu, J.; Wang, X.; Xue, W.; Gong, H. Org. Chem. Front. 2015, 2, 1411.
doi: 10.1039/C5QO00224A pmid: 24905555 |
|
|
(e) Zhang, W.; Dai, J.; Xu, H. Chin. J. Org. Chem. 2015, 35, 1820. (in Chinese)
doi: 10.6023/cjoc201503007 pmid: 24905555 |
|
|
(张文曼, 戴建军, 许华建, 有机化学, 2015, 35, 1820.)
doi: 10.6023/cjoc201503007 pmid: 24905555 |
|
| [4] |
(a) Quan, M.; Tang, L.; Shen, J.; Yang, G.; Zhang, W. Chem. Commun. 2017, 53, 609.
doi: 10.1039/C6CC08759K pmid: 29884893 |
|
(b) Quan, M.; Wang, X.; Wu, L.; Gridnev, I. D.; Yang, G.; Zhang, W. Nat. Commun. 2018, 9, 2258.
doi: 10.1038/s41467-018-04645-3 pmid: 29884893 |
|
|
(c) Wang, X.; Quan, M.; Xie, F.; Yang, G.; Zhang, W. Tetrahedron Lett. 2018, 59, 1573.
doi: 10.1016/j.tetlet.2018.03.026 pmid: 29884893 |
|
|
(d) Lv, X.-Y.; Fan, C.; Xiao, L.-J.; Xie, J.-H.; Zhou, Q.-L. CCS Chem. 2019, 1, 328.
doi: 10.31635/ccschem.019.20190026 pmid: 29884893 |
|
|
(e) Zhang, Y.; He, J.; Song, P.; Wang, Y.; Zhu, S. CCS Chem. 2020, 2, 2259.
pmid: 29884893 |
|
|
(f) Li, Z.; Wu, D.; Ding, C.; Yin, G. CCS Chem. 2020, 2, 576.
pmid: 29884893 |
|
|
(g) Wang, Z.-C.; Gao, J.; Cai, Y.; Ye, X.; Shi, S.-L. CCS Chem. 2021, 3, 1445.
doi: 10.31635/ccschem.020.202000356 pmid: 29884893 |
|
|
(h) You, C.; Li, X.; Gong, Q.; Wen, J.; Zhang, X. J. Am. Chem. Soc. 2019, 141, 14560.
doi: 10.1021/jacs.9b07957 pmid: 29884893 |
|
|
(i) Li, B.; Chen, J.; Zhang, Z.; Gridnev, I. D.; Zhang, W. Angew. Chem., Int. Ed. 2019, 58, 7329.
doi: 10.1002/anie.v58.22 pmid: 29884893 |
|
|
(j) Liu, D.; Li, B.; Chen, J.; Gridnev, I. D.; Yan, D.; Zhang, W.; Nat. Commun. 2020, 11, 5935.
doi: 10.1038/s41467-020-19807-5 pmid: 29884893 |
|
|
(k) Hu, Y.; Chen, J.; Li, B.; Zhang, Z.; Gridnev, I. D.; Zhang, W. Angew. Chem., Int. Ed. 2020, 59, 5371.
doi: 10.1002/anie.v59.13 pmid: 29884893 |
|
|
(l) Chen, J.; Zhang, W. Chin. J. Org. Chem. 2020, 40, 4372. (in Chinese)
doi: 10.6023/cjoc202000086 pmid: 29884893 |
|
|
(陈建中, 张万斌, 有机化学, 2020, 40, 4372.)
doi: 10.6023/cjoc202000086 pmid: 29884893 |
|
|
(m) Li, B.; Liu, D.; Hu, Y.; Chen, J.; Zhang, Z.; Zhang, W. Eur. J. Org. Chem. 2021, 3421.
pmid: 29884893 |
|
|
(n) Zhu, C.; Yue, H.; Nikolaienko, P.; Rueping, M. CCS Chem. 2020, 2, 179.
doi: 10.31635/ccschem.020.201900112 pmid: 29884893 |
|
|
(o) Han, X.-W.; Zhang, T.; Yao, W.-W.; Chen, H.; Ye, M. CCS Chem. 2020, 2, 955.
pmid: 29884893 |
|
|
(p) Ding, D.; Dong, H.; Wang, C. CCS Chem. 2021, 3, 718.
pmid: 29884893 |
|
|
(q) Zheng, Y.-L.; Ye, M. Chin. J. Chem. 2020, 38, 489.
doi: 10.1002/cjoc.v38.5 pmid: 29884893 |
|
|
(r) Li, Y.-Q.; Li, F.; Shi, S.-L. Chin. J. Chem. 2020, 38, 1035.
doi: 10.1002/cjoc.v38.10 pmid: 29884893 |
|
|
(s) Gan, Y.; Zhang, N.; Huang, S.; Liu, Y. Chin. J. Chem. 2020, 38, 1686.
doi: 10.1002/cjoc.v38.12 pmid: 29884893 |
|
|
(t) Xiao, C.; Xiao, W. Chin. J. Org. Chem. 2020, 40, 3004. (in Chinese)
doi: 10.6023/cjoc202000059 pmid: 29884893 |
|
|
(肖聪, 肖文精, 有机化学, 2020, 40, 3004.)
doi: 10.6023/cjoc202000059 pmid: 29884893 |
|
|
(u) Xu, G.-L.; Liu, C.-Y.; Pang, X.; Liu, X.-Y.; Shu, X.-Z. CCS Chem. 2021, 3, 1147.
pmid: 29884893 |
|
|
(v) Shao, P.; Yu, T.; Lu, H.; Xu, P.-F.; Wei, H. CCS Chem. 2020, 2, 1862.
pmid: 29884893 |
|
|
(w) Shen, H.-C.; Chen, Y.; Zhang, Y.; Jiang, H.-M.; Zhang, W.-Q.; Li, W.-A.; Sayed, M.; Zhang, X.; Wu, Y.-D.; Gong, L.-Z. CCS Chem. 2021, 3, 421.
pmid: 29884893 |
|
|
(x) Zhang, H.; Jia, Y. Chin. J. Org. Chem. 2021, 41, 1749. (in Chinese)
doi: 10.6023/cjoc202100027 pmid: 29884893 |
|
|
(张晗月, 贾义霞, 有机化学, 2021, 41, 1749.)
doi: 10.6023/cjoc202100027 pmid: 29884893 |
|
| [5] |
(a) Tamaru, Y. Modern Organonickel Chemistry, Wiley-VCH, Weinheim, 2005.
pmid: 32491839 |
|
(b) Ogoshi, S. Nickel Catalysis in Organic Synthesis: Methods and Reactions, Wiley-VCH, Weinheim, 2020.
pmid: 32491839 |
|
|
(c) Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Nature 2014, 509, 299.
doi: 10.1038/nature13274 pmid: 32491839 |
|
|
(d) Ananikov, V. P. ACS Catal. 2015, 5, 1964.
doi: 10.1021/acscatal.5b00072 pmid: 32491839 |
|
|
(e) Butt, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929.
doi: 10.1039/C5CS00144G pmid: 32491839 |
|
|
(f) Choi, J.; Fu, G. C. Science 2017, 356, eaaf7230.
doi: 10.1126/science.aaf7230 pmid: 32491839 |
|
|
(g) Fu, G. C. ACS Cent. Sci. 2017, 3, 692.
doi: 10.1021/acscentsci.7b00212 pmid: 32491839 |
|
|
(h) Ruan, L.; Dong, Z.; Chen, C.; Wu, S.; Sun, J. Chin. J. Org. Chem. 2017, 37, 2544. (in Chinese)
pmid: 32491839 |
|
|
(阮利衡, 董振诚, 陈春欣, 吴爽, 孙京, 有机化学, 2017, 37, 2544.)
doi: 10.6023/cjoc201704051 pmid: 32491839 |
|
|
(i) Zhang, Z.; Butt, N. A.; Zhou, M.; Liu, D.; Zhang, W. Chin. J. Chem. 2018, 36, 443.
doi: 10.1002/cjoc.v36.5 pmid: 32491839 |
|
|
(j) Quan, M.; Wu, L.; Yang, G.; Zhang, W. Chem. Commun. 2018, 54, 10394.
doi: 10.1039/C8CC04932G pmid: 32491839 |
|
|
(k) Liu, Y.; Bandini, M. Chin. J. Chem. 2019, 37, 431.
doi: 10.1002/cjoc.v37.5 pmid: 32491839 |
|
|
(l) Chen, J.; Butt, N. A.; Zhang, W. Res. Chem. Intermed. 2019, 45, 5959.
doi: 10.1007/s11164-019-04013-w pmid: 32491839 |
|
|
(m) Fu, L.; Greßies, S.; Chen, P.; Liu, G. Chin. J. Chem. 2020, 38, 91.
doi: 10.1002/cjoc.v38.1 pmid: 32491839 |
|
|
(n) Liu, Y.-H.; Xia, Y.-N.; Shi, B.-F. Chin. J. Chem. 2020, 38, 635.
doi: 10.1002/cjoc.v38.6 pmid: 32491839 |
|
|
(o) Liu, Y.; Dong, X.-Q.; Zhang, X. Chin. J. Org. Chem. 2020, 40, 1096. (in Chinese)
doi: 10.6023/cjoc201912025 pmid: 32491839 |
|
|
(刘元华, 董秀琴, 张绪穆, 有机化学, 2020, 40, 1096.)
doi: 10.6023/cjoc201912025 pmid: 32491839 |
|
|
(p) Cheng, L.; Zhou, Q. Acta Chim. Sinica 2020, 78, 1017. (in Chinese)
doi: 10.6023/A20070335 pmid: 32491839 |
|
|
(程磊, 周其林, 化学学报, 2020, 78, 1017.)
doi: 10.6023/A20070335 pmid: 32491839 |
|
|
(q) Clevenger, A. L.; Stolley, R. M.; Aderibigbe, J.; Louie, J. Chem. Rev. 2020, 120, 6124.
doi: 10.1021/acs.chemrev.9b00682 pmid: 32491839 |
|
|
(r) Li, Z. L.; Jin, J.; Huang, S. H. Chin. J. Org. Chem. 2020, 40, 563. (in Chinese)
doi: 10.6023/cjoc201910031 pmid: 32491839 |
|
|
(李祯龙, 金健, 黄莎华, 有机化学, 2020, 40, 563.)
doi: 10.6023/cjoc201910031 pmid: 32491839 |
|
|
(s) Dai, H.; Wu, F.; Bai, D. Chin. J. Org. Chem. 2020, 40, 1423. (in Chinese)
doi: 10.6023/cjoc202002035 pmid: 32491839 |
|
|
(代洪雪, 吴芬, 白大昌, 有机化学, 2020, 40, 1423.)
doi: 10.6023/cjoc202002035 pmid: 32491839 |
|
|
(t) Chen, S.; Zhao, Y. Chin. J. Org. Chem. 2020, 40, 3078. (in Chinese)
doi: 10.6023/cjoc202005072 pmid: 32491839 |
|
|
(陈思, 赵延川, 有机化学, 2020, 40, 3078.)
doi: 10.6023/cjoc202005072 pmid: 32491839 |
|
|
(u) Luo, Y.-C.; Xu, C.; Zhang, X. Chin. J. Chem. 2020, 38, 1371.
doi: 10.1002/cjoc.v38.11 pmid: 32491839 |
|
|
(v) Xue, W.; Jia, X.; Wang, X.; Tao, X.; Yin, Z.; Gong, H. Chem. Soc. Rev. 2021, 50, 4162.
doi: 10.1039/D0CS01107J pmid: 32491839 |
|
|
(w) Xie, J.-Q.; Liang, R.-X.; Jia, Y.-X. Chin. J. Chem. 2021, 39, 710.
doi: 10.1002/cjoc.v39.3 pmid: 32491839 |
|
| [6] |
(a) Rosen, B. M.; Quasdorf, K. W.; Wilson, D. A.; Zhang, N.; Resmerita, A.-M.; Garg, N.; Percec, K. V. Chem. Rev. 2011, 111, 1346.
pmid: 25157613 |
|
(b) Cornella, J.; Zarate, C.; Martin, R. Chem. Soc. Rev. 2014, 43, 8081.
doi: 10.1039/c4cs00206g pmid: 25157613 |
|
|
(c) Tobisu, M.; Chatani, N. Acc. Chem. Res. 2015, 48, 1717.
doi: 10.1021/acs.accounts.5b00051 pmid: 25157613 |
|
|
(d) Tollefson, E. J.; Hanna, L. E.; Jarvo, E. R. Acc. Chem. Res. 2015, 48, 2344.
doi: 10.1021/acs.accounts.5b00223 pmid: 25157613 |
|
|
(e) Su, B.; Cao, Z.-C.; Shi, Z.-J. Acc. Chem. Res. 2015, 48, 886.
doi: 10.1021/ar500345f pmid: 25157613 |
|
|
(f) Zarate, C.; van Gemmeren, M.; Somerville, R. J.; Martin, R. Adv. Organomet. Chem. 2016, 66, 143.
pmid: 25157613 |
|
|
(g) Pound, S. M.; Watson, M. P. Chem. Commun. 2018, 54, 12286.
doi: 10.1039/C8CC07093H pmid: 25157613 |
|
|
(h) Liu, J.; Ye, Y.; Sessler, J. L.; Gong, H. Acc. Chem. Res. 2020, 53, 1833.
doi: 10.1021/acs.accounts.0c00291 pmid: 25157613 |
|
|
(i) Xu, J.; Bercher, O. P.; Talley, M. R.; Watson, M. P. ACS Catal. 2021, 11, 1604.
doi: 10.1021/acscatal.0c05484 pmid: 25157613 |
|
| [7] |
(a) Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395.
doi: 10.1021/cr9409804 |
|
(b) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921.
doi: 10.1021/cr020027w |
|
|
(c) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258.
|
|
|
(d) Diéguez, M.; Pàmies, O. Acc. Chem. Res. 2010, 43, 312.
doi: 10.1021/ar9002152 |
|
|
(e) Weaver, J. D.; Recio, A.; Grenning, A. J.; Tunge, J. A. Chem. Rev. 2011, 111, 1846.
doi: 10.1021/cr1002744 |
|
|
(f) Huo, X.; Yang, G.; Liu, D.; Liu, Y.; Gridnev, I. D.; Zhang, W. Angew. Chem., Int. Ed. 2014, 53, 6776.
doi: 10.1002/anie.201403410 |
|
|
(g) Huo, X.; He, R.; Zhang, X.; Zhang, W. J. Am. Chem. Soc. 2016, 138, 11093.
doi: 10.1021/jacs.6b06156 |
|
|
(h) Tang, H.; Huo, X.; Meng, Q.; Zhang, W. Acta Chim. Sinica 2016, 74, 219. (in Chinese)
doi: 10.6023/A16020078 |
|
|
(汤溟淏, 霍小红, 孟庆华, 张万斌, 化学学报, 2016, 74, 219.)
|
|
|
(i) Huo, X.; He, R.; Fu, J.; Zhang, J.; Yang, G.; Zhang, W. J. Am. Chem. Soc. 2017, 139, 9819.
doi: 10.1021/jacs.7b05460 |
|
|
(j) Huo, X.; Zhang, J.; Fu, J.; Zhang, W. J. Am. Chem. Soc. 2018, 140, 2080.
doi: 10.1021/jacs.8b00187 |
|
|
(k) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76, 838. (in Chinese)
doi: 10.6023/A18060237 |
|
|
(张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报, 2018, 76, 838.)
doi: 10.6023/A18060237 |
|
|
(l) Li, Z.; Zheng, J.; Li, C.; Wu, W.; Jiang, H. Chin. J. Chem. 2019, 37, 140.
doi: 10.1002/cjoc.v37.2 |
|
|
(m) Wang, R.; Luan, Y.; Ye, M. Chin. J. Chem. 2019, 37, 720.
doi: 10.1002/cjoc.v37.7 |
|
|
(n) Yao, K.; Liu, H.; Yuan, Q.; Liu, Y.; Liu, D.; Zhang, W. Acta Chim. Sinica 2019, 77, 993. (in Chinese)
doi: 10.6023/A19060210 |
|
|
(姚坤, 刘浩, 袁乾家, 刘燕刚, 刘德龙, 张万斌, 化学学报, 2019, 77, 993.)
doi: 10.6023/A19060210 |
|
|
(o) Zhang, H.-H.; Yu, S. Acta Chim. Sinica 2019, 77, 832. (in Chinese)
doi: 10.6023/A19050177 |
|
|
(张洪浩, 俞寿云, 化学学报, 2019, 77, 832.)
doi: 10.6023/A19050177 |
|
|
(p) Zhang, H.; Gu, Q.; You, S. Chin. J. Org. Chem. 2019, 39, 15. (in Chinese)
|
|
|
(张慧君, 顾庆, 游书力, 有机化学, 2019, 39, 15.)
doi: 10.6023/cjoc201809037 |
|
|
(q) Huang, L.; Cai, Y.; Zhang, H.-J.; Zheng, C.; Dai, L.-X.; You, S.-L. CCS Chem. 2019, 1, 106.
|
|
|
(r) Wang, R.-Q.; Shen, C.; Cheng, X.; Wang, Z.-F.; Tao, H.-Y.; Dong, X.-Q.; Wang, C.-J. Chin. J. Chem. 2020, 38, 807.
doi: 10.1002/cjoc.v38.8 |
|
|
(s) He, R.; Huo, X.; Zhao, L.; Wang, F.; Jiang, L.; Liao, J.; Zhang, W. J. Am. Chem. Soc. 2020, 142, 8097.
doi: 10.1021/jacs.0c02150 |
|
|
(t) Wang, Y.; Luo, S. Chin. J. Org. Chem. 2020, 40, 2161. (in Chinese)
doi: 10.6023/cjoc202000038 |
|
|
(王娅宁, 罗三中, 有机化学, 2020, 40, 2161.)
doi: 10.6023/cjoc202000038 |
|
|
(u) Ju, C.; Wu, Z.; Li, Y.; Zhang, W. Chin. J. Org. Chem. 2020, 40, 3925. (in Chinese)
doi: 10.6023/cjoc202004025 |
|
|
(居辰阳, 吴正兴, 李云艺, 张万斌, 有机化学, 2020, 40, 3925.)
doi: 10.6023/cjoc202004025 |
|
|
(v) Li, G.; Huo, X.; Jiang, X.; Zhang, W. Chem. Soc. Rev. 2020, 49, 2060.
doi: 10.1039/C9CS00400A |
|
|
(w) Ma, X.; Yu, J.; Wang, Z.; Zhang, Y.; Zhou, Q. Chin. J. Org. Chem. 2020, 40, 2669. (in Chinese)
doi: 10.6023/cjoc202005013 |
|
|
(马献涛, 于静, 王子龙, 张赟, 周秋菊, 有机化学, 2020, 40, 2669.)
doi: 10.6023/cjoc202005013 |
|
|
(x) Tian, F.; Zhang, J.; Yang, W.; Deng, W. Chin. J. Org. Chem. 2020, 40, 3262. (in Chinese)
doi: 10.6023/cjoc202005008 |
|
|
(田飞, 张键, 杨武林, 邓卫平, 有机化学, 2020, 40, 3262.)
doi: 10.6023/cjoc202005008 |
|
|
(y) Huo, X.; Zhao, L.; Luo, Y.; Wu, Y.; Sun, Y.; Li, G.; Gridneva, T.; Zhang, J.; Ye, Y.; Zhang, W. CCS Chem. 2021, 3, 1933.
|
|
|
(z) Xiao, J.; Xu, H.; Huo, X.; Zhang, W.; Ma, S. Chin. J. Chem. 2021, 39, 1958.
doi: 10.1002/cjoc.v39.7 |
|
| [8] |
Yanagisawa, A.; Nomura, N.; Habaue, S.; Yamamoto, H. Tetrahedron Lett. 1989, 30, 6409.
doi: 10.1016/S0040-4039(01)93908-5 |
| [9] |
Nagel, U.; Nedden, H. G. Inorg. Chim. Acta 1998, 269, 34.
doi: 10.1016/S0020-1693(97)05770-8 |
| [10] |
(a) Novak, A.; Fryatt, R.; Woodward, S. C. R. Chim. 2007, 10, 206.
doi: 10.1016/j.crci.2006.10.008 |
|
(b) Novak, A.; Calhorda, M. J.; Costa, P. J.; Woodward, S. Eur. J. Org. Chem. 2009, 898.
|
|
| [11] |
Sumida, Y.; Hayashi, S.; Hirano, K.; Yorimitsu, H.; Oshima, K. Org. Lett. 2008, 10, 1629.
doi: 10.1021/ol800335v pmid: 18355073 |
| [12] |
Matsubara, R.; Jamison, T. F. J. Am. Chem. Soc. 2010, 132, 6880.
doi: 10.1021/ja101186p pmid: 20433144 |
| [13] |
Ho, C.-Y.; Jamison, T. F. Angew. Chem., Int. Ed. 2007, 46, 782.
doi: 10.1002/(ISSN)1521-3773 |
| [14] |
Wang, Z.-J.; Zheng, S.; Romero, E.; Matsui, J. K.; Molander, G. A. Org. Lett. 2019, 21, 6543.
doi: 10.1021/acs.orglett.9b02473 pmid: 31390217 |
| [15] |
Tran, V. T.; Li, Z.-Q.; Gallagher, T. J.; Derosa, J.; Liu, P.; Engle, K. M. Angew. Chem., Int. Ed. 2020, 59, 7029.
doi: 10.1002/anie.v59.18 |
| [16] |
Eno, M. S.; Lu, A.; Morken, J. P. J. Am. Chem. Soc. 2016, 138, 7824.
doi: 10.1021/jacs.6b03384 |
| [17] |
Guan, B.-T.; Xiang, S.-K.; Wang, B.-Q.; Sun, Z.-P.; Wang, Y.; Zhao, K.-Q.; Shi, Z.-J. J. Am. Chem. Soc. 2008, 130, 3268.
doi: 10.1021/ja710944j |
| [18] |
Yu, D.-G.; Wang, X.; Zhu, R.-Y.; Luo, S.; Zhang, X.-B.; Wang, B.-Q.; Wang, L.; Shi, Z.-J. J. Am. Chem. Soc. 2012, 134, 14638.
doi: 10.1021/ja307045r |
| [19] |
Huang, Y. K.; Li, G.; Huang, W.-P.; Yu, D.-G.; Shi, Z.-J. Chem. Commun. 2011, 47, 7224.
doi: 10.1039/c1cc11193k |
| [20] |
Barreiro, E. J.; Kümmerle, A. E.; Fraga, C. A. M. Chem. Rev. 2011, 111, 5215.
doi: 10.1021/cr200060g pmid: 21631125 |
| [21] |
Liang, Z.; Xue, W.; Lin, K.; Gong, H. Org. Lett. 2014, 16, 5620.
doi: 10.1021/ol502682q |
| [22] |
Xu, H.; Zhao, C.; Qian, Q.; Deng, W.; Gong, H. Chem. Sci. 2013, 4, 4022.
doi: 10.1039/c3sc51098k |
| [23] |
Wang, J.; Zhao, J.; Gong, H. Chem. Commun. 2017, 53, 10180.
doi: 10.1039/C7CC06106D |
| [24] |
Komeyama, K.; Michiyuki, T.; Osaka, I. ACS Catal. 2019, 9, 9285.
doi: 10.1021/acscatal.9b03352 |
| [25] |
Smith, R. T.; Zhang, X.; Rincon, J. A.; Agejas, J.; Mateos, C.; Barberis, M.; García-Cerrada, S.; de Frutos, O.; MacMillan, D. W. C. J. Am. Chem. Soc. 2018, 140, 17433.
doi: 10.1021/jacs.8b12025 |
| [26] |
Le, C.; Chen, T. Q.; Liang, T.; Zhang, P.; MacMillan, D. W. C. Science 2018, 360, 1010.
doi: 10.1126/science.aat4133 |
| [27] |
(a) Chatgilialoglu, C. Acc. Chem. Res. 1992, 25, 188.
doi: 10.1021/ar00016a003 pmid: 29938502 |
|
(b) Chatgilialoglu, C.; Ferreri, C.; Landais, Y.; Timokhin, V. I. Chem. Rev. 2018, 118, 6516.
doi: 10.1021/acs.chemrev.8b00109 pmid: 29938502 |
|
| [28] |
Durandetti, M.; Devaud, M.; Perichon, J. New J. Chem. 1996, 20, 659.
|
| [29] |
Gutierrez, O.; Tellis, J. C.; Primer, D. N.; Molander, G. A.; Kozlowski, M. C. J. Am. Chem. Soc. 2015, 137, 4896.
doi: 10.1021/ja513079r pmid: 25836634 |
| [30] |
(a) Lin, X.; Phillips, D. L. J. Org. Chem. 2008, 73, 3680.
doi: 10.1021/jo702497p pmid: 23865460 |
|
(b) Breitenfeld, J.; Ruiz, J.; Wodrich, M. D.; Hu, X. J. Am. Chem. Soc. 2013, 135, 12004.
doi: 10.1021/ja4051923 pmid: 23865460 |
|
| [31] |
Dai, Y.; Wu, F.; Zang, Z.; You, H.; Gong, H. Chem.-Eur. J. 2012, 18, 808.
doi: 10.1002/chem.v18.3 |
| [32] |
Moragas, T.; Cornella, J.; Martin, R. J. Am. Chem. Soc. 2014, 136, 17702.
doi: 10.1021/ja509077a pmid: 25473825 |
| [33] |
Chen, H.; Jia, X.; Yu, Y.; Qian, Q.; Gong, H. Angew. Chem., Int. Ed. 2017, 56, 13103.
|
| [34] |
Ackerman, L. K. G.; Anka-Lufford, L. L.; Naodovic, M.; Weix, D. J. Chem. Sci. 2015, 6, 1115.
doi: 10.1039/C4SC03106G pmid: 25685312 |
| [35] |
Yan, X.-B.; Li, C.-L.; Jin, W.-J.; Guo, P.; Shu, X.-Z. Chem. Sci. 2018, 9, 4529.
doi: 10.1039/C8SC00609A |
| [36] |
Konev, M. O.; Hanna, L. E.; Jarvo, E. R. Angew. Chem., Int. Ed. 2016, 55, 6730.
doi: 10.1002/anie.v55.23 |
| [37] |
Pan, Y.; Gong, Y.; Song, Y.; Tong, W.; Gong, H. Org. Biomol. Chem. 2019, 17, 4230.
doi: 10.1039/C9OB00628A |
| [38] |
Guo, P.; Wang, K.; Jin, W.-J.; Xie, H.; Qi, L.; Liu, X.-Y.; Shu, X.-Z. J. Am. Chem. Soc. 2021, 143, 513.
doi: 10.1021/jacs.0c12462 |
| [39] |
(a) Diccianni, J. B.; Diao, T. Trends Chem. 2019, 1, 830.
doi: 10.1016/j.trechm.2019.08.004 pmid: 24820397 |
|
(b) Everson, D. A.; Weix, D. J. J. Org. Chem. 2014, 79, 4793.
doi: 10.1021/jo500507s pmid: 24820397 |
|
| [40] |
Kariofillis, S. K.; Shields, B. J.; Tekle-Smith, M. A.; Zacuto, M. J.; Doyle, A. G. J. Am. Chem. Soc. 2020, 142, 7683.
doi: 10.1021/jacs.0c02805 pmid: 32275411 |
| [41] |
Suga, T.; Ukaji, Y. Org. Lett. 2018, 20, 7846.
doi: 10.1021/acs.orglett.8b03367 |
| [42] |
(a) Biswas, S.; Weix, D. J. J. Am. Chem. Soc. 2013, 135, 16192.
doi: 10.1021/ja407589e pmid: 25836634 |
|
(b) Gutierrez, O.; Tellis, J. C.; Primer, D. N.; Molander, G. A.; Kozlowski, M. C. J. Am. Chem. Soc. 2015, 137, 4896.
doi: 10.1021/ja513079r pmid: 25836634 |
|
|
(c) Wang, X.; Ma, G.; Peng, Y.; Pitsch, C. E.; Moll, B. J.; Ly, T. D.; Wang, X.; Gong, H. J. Am. Chem. Soc. 2018, 140, 14490.
doi: 10.1021/jacs.8b09473 pmid: 25836634 |
|
| [43] |
Chenniappan, V. K.; Peck, D.; Rahaim, R. Tetrahedron Lett. 2020, 61, 151729.
doi: 10.1016/j.tetlet.2020.151729 |
| [44] |
Jia, X.-G.; Guo, P.; Duan, J.; Shu, X.-Z. Chem. Sci. 2018, 9, 640.
doi: 10.1039/C7SC03140H |
| [45] |
van Gemmeren, M.; Börjesson, M.; Tortajada, A.; Sun, S.-Z.; Okura, K.; Martin, R. Angew. Chem., Int. Ed. 2017, 56, 6558.
doi: 10.1002/anie.201702857 |
| [46] |
Consiglio, G.; Morandini, F.; Piccolo, O. Helv. Chim. Acta 1980, 63, 987.
doi: 10.1002/(ISSN)1522-2675 |
| [47] |
(a) Consiglio, G.; Morandini, F.; Piccolo, O. J. Chem. Soc., Chem. Commun. 1983, 112.
|
|
(b) Consiglio, G.; Piccolo, O.; Roncetti, L.; Morandini, F. Tetrahedron 1986, 42, 2043.
doi: 10.1016/S0040-4020(01)87621-3 |
|
|
(c) Indolese, A. F.; Consiglio, G. Organometallics 1994, 13, 2230.
doi: 10.1021/om00018a016 |
|
|
(d) Consiglio, G.; Indolese, A. Organometallics 1991, 10, 3425.
doi: 10.1021/om00056a007 |
|
| [48] |
Chung, K.-G.; Miyake, Y.; Uemura, S. J. Chem. Soc., 2000, 2725.
|
| [49] |
Hiyama, T.; Wakasa, N. Tetrahedron Lett. 1985, 26, 3259.
doi: 10.1016/S0040-4039(00)98166-8 |
| [50] |
Nomura, N.; RajanBabu, T. V. Tetrahedron Lett. 1997, 38, 1713.
|
| [51] |
Didiuk, M. T.; Morken, J. P.; Hoveyda, A. H. Tetrahedron 1998, 54, 1117.
doi: 10.1016/S0040-4020(97)10212-5 |
| [52] |
(a) Eliel, E. L.; Wilen, S. H. Stereochemistry of Organic Compounds, John Wiley & Sons, New York, 1994.
|
|
(b) Walsh, P. J.; Kowzlowski, M. C. Fundamentals of Asymmetric Catalysis, University Science Books, Sausalito, CA, 2009.
|
|
| [53] |
Taylor, B. L. H.; Swift, E. C.; Waetzig, J. D.; Jarvo, E. R. J. Am. Chem. Soc. 2011, 133, 389.
doi: 10.1021/ja108547u pmid: 21155567 |
| [54] |
Yonova, I. M.; Johnson, A. G.; Osborne, C. A.; Moore, C. E.; Morrissette, N. S.; Jarvo, E. R. Angew. Chem., Int. Ed. 2014, 53, 2422.
doi: 10.1002/anie.201308666 |
| [55] |
(a) Greene, M. A.; Yonova, I. M.; Williams, F. J.; Jarvo, E. R. Org. Lett. 2012, 14, 4293.
doi: 10.1021/ol300891k |
|
(b) Taylor, B. L. H.; Harris, M. R.; Jarvo, E. R. Angew. Chem., Int. Ed. 2012, 51, 7790.
doi: 10.1002/anie.201202527 |
|
| [56] |
Tollefson, E. J.; Dawson, D. D.; Osborne, C. A.; Jarvo, E. R. J. Am. Chem. Soc. 2014, 136, 14951.
doi: 10.1021/ja5076426 pmid: 25308512 |
| [57] |
Dawson, D. D.; Jarvo, E. R. Org. Process Res. Dev. 2015, 19, 1356.
pmid: 27458328 |
| [58] |
(a) Chen, P.-P.; Lucas, E. L.; Greene, M. A.; Zhang, S.-Q.; Tollefson, E. J.; Erickson, L. W.; Taylor, B. L. H.; Jarvo, E. R.; Hong, X. J. Am. Chem. Soc. 2019, 141, 5835.
doi: 10.1021/jacs.9b00097 pmid: 31953874 |
|
(b) Dawson, D. D.; Oswald, V. F.; Borovik, A. S.; Jarvo, E. R. Chem.-Eur. J. 2020, 26, 3044.
doi: 10.1002/chem.202000215 pmid: 31953874 |
|
| [59] |
Wisniewska, H. M.; Swift, E. C.; Jarvo, E. R. J. Am. Chem. Soc. 2013, 135, 9083.
doi: 10.1021/ja4034999 pmid: 23751004 |
| [60] |
Do, H.-Q.; Chandrashekar, E. R. R., Fu, G. C. J. Am. Chem. Soc. 2013, 135, 16288.
doi: 10.1021/ja408561b |
| [61] |
Yang, B.; Wang, Z.-X. J. Org. Chem. 2017, 82, 4542.
doi: 10.1021/acs.joc.6b02564 pmid: 28472890 |
| [62] |
Nielsen, D. K.; Doyle, A. G. Angew. Chem., Int. Ed. 2011, 50, 6056.
doi: 10.1002/anie.v50.27 |
| [63] |
(a) Jørgensen, K. A.; Schioett, B. Chem. Rev. 1990, 90, 1483.
doi: 10.1021/cr00106a006 pmid: 12837057 |
|
(b) de Bruin, B.; Budzelaar, P. H. M.; Gal, A. W. Angew. Chem., Int. Ed. 2004, 43, 4142.
doi: 10.1002/(ISSN)1521-3773 pmid: 12837057 |
|
|
(c) Lenarda, M.; Pahor, N. B.; Calligaris, M.; Graziani, M.; Randaccio, L. J. Chem. Soc., Dalton Trans. 1978, 279.
pmid: 12837057 |
|
|
(d) Schlodder, R.; Ibers, J.; Lenarda, M.; Graziani, M. J. Am. Chem. Soc. 1974, 96, 6893.
doi: 10.1021/ja00829a014 pmid: 12837057 |
|
|
(e) Molinaro, C.; Jamison, T. J. Am. Chem. Soc. 2003, 125, 8076.
pmid: 12837057 |
|
| [64] |
Harris, M. R.; Hanna, L. E.; Greene, M. A.; Moore, C. E.; Jarvo, E. R. J. Am. Chem. Soc. 2013, 135, 3303.
doi: 10.1021/ja311783k |
| [65] |
Zhang, S.-Q.; Taylor, B. L. H.; Ji, C.-L.; Gao, Y.; Harris, M. R.; Hanna, L. E.; Jarvo, E. R.; Houk, K. N.; Hong, X. J. Am. Chem. Soc. 2017, 139, 12994.
doi: 10.1021/jacs.7b04973 |
| [66] |
Zhou, Q.; Srinivas, H. D.; Dasgupta, S.; Watson, M. P. J. Am. Chem. Soc. 2013, 135, 3307.
doi: 10.1021/ja312087x pmid: 23425080 |
| [67] |
Chung, K.-G.; Miyake, Y.; Uemura, S. J. Chem. Soc., Perkin Trans. 1 2000, 15.
|
| [68] |
Chen, H.; Deng, M.-Z. J. Organomet. Chem. 2000, 603, 189.
doi: 10.1016/S0022-328X(00)00164-9 |
| [69] |
(a) Kobayashi, Y.; Tokoro, Y.; Watatani, K. Tetrahedron Lett. 1998, 39, 7537.
doi: 10.1016/S0040-4039(98)01639-6 |
|
(b) Kobayashi, Y.; Mizojiri, R.; Ikeda, E. J. Org. Chem. 1996, 61, 5391.
doi: 10.1021/jo960458c |
|
|
(c) Kobayashi, Y.; Watatani, K.; Kikori, Y.; Mizojiri, R. Tetrahedron Lett. 1996, 37, 6125.
doi: 10.1016/0040-4039(96)01291-9 |
|
|
(d) Kobayashi, Y.; Takahisa, E.; Usmani, S. B. Tetrahedron Lett. 1998, 39, 597.
doi: 10.1016/S0040-4039(97)10654-2 |
|
|
(e) Kobayashi, Y.; Tokoro, Y.; Watatani, K. Eur. J. Org. Chem. 2000, 3825.
|
|
| [70] |
Srinivas, H. D.; Zhou, Q.; Watson, M. P. Org. Lett. 2014, 16, 3596.
doi: 10.1021/ol5016724 pmid: 24927013 |
| [71] |
Cobb, K. M.; Rabb-Lynch, J. M.; Hoerrner, M. E.; Manders, A.; Zhou, Q.; Watson, M. P. Org. Lett. 2017, 19, 4355.
doi: 10.1021/acs.orglett.7b02063 |
| [72] |
Gao, M.; Sun, D.; Gong, H. Org. Lett. 2019, 21, 1645.
doi: 10.1021/acs.orglett.9b00174 |
| [73] |
Song, F.; Wang, F.; Guo, L.; Feng, X.; Zhang, Y.; Chu, L. Angew. Chem., Int. Ed. 2020, 59, 177.
doi: 10.1002/anie.v59.1 |
| [74] |
Zhang, X.; MacMillan, D. W. C. J. Am. Chem. Soc. 2016, 138, 13862.
doi: 10.1021/jacs.6b09533 |
| [75] |
Lin, Z.; Jin, Y.; Hu, W.; Wang, C. Chem. Sci. 2021, 12, 6712.
doi: 10.1039/D1SC01115D |
| [76] |
Zhao, Y.; Weix, D. J. J. Am. Chem. Soc. 2014, 136, 48.
doi: 10.1021/ja410704d |
| [77] |
Zhao, Y.; Weix, D. J. J. Am. Chem. Soc. 2015, 137, 3237.
doi: 10.1021/jacs.5b01909 |
| [78] |
Parasram, M.; Shields, B. J.; Ahmad, O.; Knauber, T.; Doyle, A. G. ACS Catal. 2020, 10, 5821.
doi: 10.1021/acscatal.0c01199 pmid: 32747870 |
| [79] |
Hu, P.; Chi, H. M.; DeBacker, K. C.; Gong, X.; Keim, J. H.; Hsu, I. T.; Snyder, S. A. Nature 2019, 569, 703.
doi: 10.1038/s41586-019-1179-2 |
| [80] |
Mills, L. R.; Monteith, J. J.; dos Passos Gomes, G.; Aspuru-Guzik, A.; Rousseaux, S. A. L. J. Am. Chem. Soc. 2020, 142, 13246.
doi: 10.1021/jacs.0c06904 |
| [81] |
Mills, L. R.; Monteith, J. J.; Rousseaux, S. A. L. Chem. Commun. 2020, 56, 12538.
doi: 10.1039/D0CC05895E |
| [82] |
Ye, Y.; Chen, H.; Sessler, J. L.; Gong, H. J. Am. Chem. Soc. 2019, 141, 820.
doi: 10.1021/jacs.8b12801 |
| [83] |
Zhou, Q.; Cobb, K. M.; Tan, T.; Watson, M. P. J. Am. Chem. Soc. 2016, 138, 12057.
doi: 10.1021/jacs.6b08075 pmid: 27610831 |
| [84] |
Xu, J.; Pound, S. M.; Basch, C. H.; Duke, A. D.; Watson, M. P. ChemRxiv DOI: 10.26434/chemrxiv.14403302.v1.
doi: 10.26434/chemrxiv.14403302.v1 |
| [85] |
Xu, J.; Bercher, O. P.; Watson, M. P. J. Am. Chem. Soc. 2021, 143, 8608.
doi: 10.1021/jacs.1c03898 |
| [86] |
Wang, Z.; Yin, H.; Fu, G. C. Nature 2018, 563, 379.
doi: 10.1038/s41586-018-0669-y |
| [87] |
Wu, L.; Yang, G.; Zhang, W. CCS Chem. 2019, 1, 623.
|
| [88] |
Gomez-Bengoa, E.; Heron, N. M.; Didiuk, M. T.; Luchaco, C. A.; Hoveyda, A. H. J. Am. Chem. Soc. 1998, 120, 7649.
doi: 10.1021/ja980499l |
| [89] |
Wu, K.; Doyle, A. G. Nat. Chem. 2017, 9, 779.
doi: 10.1038/nchem.2741 |
| [90] |
Arendt, K. M.; Doyle, A. G. Angew. Chem., Int. Ed. 2015, 54, 9876.
doi: 10.1002/anie.v54.34 |
| [91] |
Lin, Z.; Lan, Y.; Wang, C. ACS Catal. 2019, 9, 775.
doi: 10.1021/acscatal.8b04348 |
| [92] |
Graham, T. J. A.; Shields, J. D.; Doyle, A. G. Chem. Sci. 2011, 2, 980.
doi: 10.1039/C1SC00026H |
| [93] |
Shields, J. D.; Ahneman, D. T.; Graham, T. J. A.; Doyle, A. G. Org. Lett. 2014, 16, 142.
doi: 10.1021/ol4031364 pmid: 24279380 |
| [94] |
Tao, X.; Chen, Y.; Guo, J.; Wang, X.; Gong, H. Chem. Sci. 2021, 12, 220.
doi: 10.1039/D0SC05452F |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [4] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [5] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [6] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [7] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [8] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [9] | 刘婷婷, 胡宇才, 沈安. 亚胺配体协同氮杂环卡宾钯配合物催化碳碳偶联反应的作用机制[J]. 有机化学, 2023, 43(2): 622-628. |
| [10] | 吴利城, 伍贤青, 曲景平, 陈宜峰. Quinim配体的探索及其在镍催化烯烃的不对称胺甲酰基-烷基化反应的应用[J]. 有机化学, 2023, 43(12): 4239-4250. |
| [11] | 赵佳怡, 葛怡聪, 何川. 不对称催化Si—H/X—H脱氢偶联构筑硅中心手性[J]. 有机化学, 2023, 43(10): 3352-3366. |
| [12] | 曾燕, 叶飞. 不对称催化构建硅立体中心化合物的新反应体系研究进展[J]. 有机化学, 2023, 43(10): 3388-3413. |
| [13] | 代增进, 张绪穆, 殷勤. 铵盐为胺源的不对称还原胺化反应研究进展[J]. 有机化学, 2022, 42(8): 2261-2274. |
| [14] | 李晖, 殷亮. 铜催化的直接型插烯反应研究进展[J]. 有机化学, 2022, 42(6): 1573-1585. |
| [15] | 吴逾诸, 申盼盼, 段文增, 马玉道. 卡宾催化对亚甲基苯醌的不对称硼化反应的研究[J]. 有机化学, 2022, 42(5): 1483-1492. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||