有机化学 ›› 2022, Vol. 42 ›› Issue (7): 2089-2097.DOI: 10.6023/cjoc202203001 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑
研究论文
收稿日期:2022-03-01
修回日期:2022-04-03
发布日期:2022-08-09
通讯作者:
孙凯
基金资助:Received:2022-03-01
Revised:2022-04-03
Published:2022-08-09
Contact:
Kai Sun
Supported by:文章分享
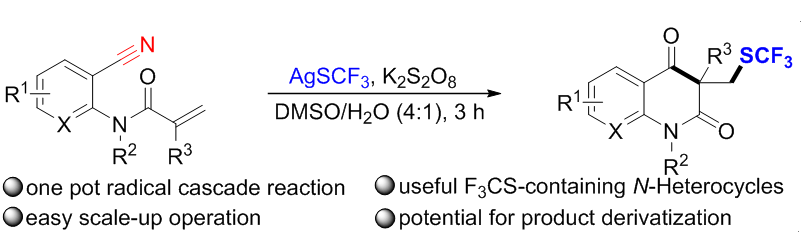
以氰基作为自由基受体, 开发了一种实用的银催化三氟甲硫化/环化/水解策略. 在中等到较高产率的条件下, 获得了各种结构多样的F3CS-取代喹啉酮类化合物. 该方法易于放大量制备, 且产物具有进一步衍生化的潜力. 因此, 对制备其他有价值的含氟砌块的氮杂环化合物很有吸引力. 机理研究表明, 该催化体系涉及自由基途径, H2O在水解过程中起着不可或缺的作用.
李猛, 孙凯. 银催化三氟甲硫基化-环化-水解: 含F3CS基喹啉酮合成[J]. 有机化学, 2022, 42(7): 2089-2097.
Meng Li, Kai Sun. Silver-Mediated Trifluoromethylthiolation-Cyclization-Hydrolysis: Access to F3CS-Containing Quinolinones[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2089-2097.
| Entry | Solvent | Oxidant | T/℃ | Yield b/% |
|---|---|---|---|---|
| 1 | DMSO | K2S2O8 | 80 | 35 |
| 2 | V(DMSO)∶V(H2O)=4∶1 | K2S2O8 | 80 | 59 |
| 3 | V(DMSO)∶V(H2O)=2∶1 | K2S2O8 | 80 | 33 |
| 4 | V(DMSO)∶V(H2O)=1∶1 | K2S2O8 | 80 | 33 |
| 5 | V(CH3CN)∶V(H2O)=4∶1 | K2S2O8 | 80 | 55 |
| 6 | V(DMF)∶V(H2O)=4∶1 | K2S2O8 | 80 | 46 |
| 7 | V(acetone)∶V(H2O)=4∶1 | K2S2O8 | 80 | 57 |
| 8 | V(dioxane)∶V(H2O)=4∶1 | K2S2O8 | 80 | 36 |
| 9 | V(EtOAc)∶V(H2O)=4∶1 | K2S2O8 | 80 | 0 |
| 10 | V(DMSO)∶V(H2O)=4∶1 | K2S2O8 | 60 | 25 |
| 11 | V(DMSO)∶V(H2O)=4∶1 | K2S2O8 | 100 | 68 |
| 12 | V(DMSO)∶V(H2O)=4∶1 | K2S2O8 | 120 | 85 |
| 13 | V(DMSO)∶V(H2O)=4∶1 | Na2S2O8 | 120 | 71 |
| 14 | V(DMSO)∶V(H2O)=4∶1 | (NH4)2S2O8 | 120 | 40 |
| 15 | V(DMSO)∶V(H2O)=4∶1 | PhI(OAc)2 | 120 | Trace |
| 16 | V(DMSO)∶V(H2O)=4∶1 | TBHP | 120 | 0 |
| 17 | V(DMSO)∶V(H2O)=4∶1 | Selectfluor | 120 | 0 |
| 18 | V(DMSO)∶V(H2O)=4∶1 | Oxone | 120 | 0 |
| Entry | Solvent | Oxidant | T/℃ | Yield b/% |
|---|---|---|---|---|
| 1 | DMSO | K2S2O8 | 80 | 35 |
| 2 | V(DMSO)∶V(H2O)=4∶1 | K2S2O8 | 80 | 59 |
| 3 | V(DMSO)∶V(H2O)=2∶1 | K2S2O8 | 80 | 33 |
| 4 | V(DMSO)∶V(H2O)=1∶1 | K2S2O8 | 80 | 33 |
| 5 | V(CH3CN)∶V(H2O)=4∶1 | K2S2O8 | 80 | 55 |
| 6 | V(DMF)∶V(H2O)=4∶1 | K2S2O8 | 80 | 46 |
| 7 | V(acetone)∶V(H2O)=4∶1 | K2S2O8 | 80 | 57 |
| 8 | V(dioxane)∶V(H2O)=4∶1 | K2S2O8 | 80 | 36 |
| 9 | V(EtOAc)∶V(H2O)=4∶1 | K2S2O8 | 80 | 0 |
| 10 | V(DMSO)∶V(H2O)=4∶1 | K2S2O8 | 60 | 25 |
| 11 | V(DMSO)∶V(H2O)=4∶1 | K2S2O8 | 100 | 68 |
| 12 | V(DMSO)∶V(H2O)=4∶1 | K2S2O8 | 120 | 85 |
| 13 | V(DMSO)∶V(H2O)=4∶1 | Na2S2O8 | 120 | 71 |
| 14 | V(DMSO)∶V(H2O)=4∶1 | (NH4)2S2O8 | 120 | 40 |
| 15 | V(DMSO)∶V(H2O)=4∶1 | PhI(OAc)2 | 120 | Trace |
| 16 | V(DMSO)∶V(H2O)=4∶1 | TBHP | 120 | 0 |
| 17 | V(DMSO)∶V(H2O)=4∶1 | Selectfluor | 120 | 0 |
| 18 | V(DMSO)∶V(H2O)=4∶1 | Oxone | 120 | 0 |
| [16] |
(c) Guo, Y.-H.; Xiang, Y.-F.; Wei, L.; Wan, J.-P. Org. Lett. 2018, 20, 3971.
doi: 10.1021/acs.orglett.8b01536 |
|
(d) Li, G.-Q.; Gan, Z.-Y.; Kong, K.-X.; Dou, X.-M.; Yang, D.-S. Adv. Synth. Catal. 2019, 361, 1808.
doi: 10.1002/adsc.201900157 |
|
|
(e) Song, S.-Z.; Meng, Y.-N.; Li, Q.; Wei, W.-T. Adv. Synth. Catal. 2020, 362, 2120.
doi: 10.1002/adsc.202000055 |
|
| [1] |
(a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
doi: 10.1126/science.1131943 |
|
(b) Hird, M. Chem. Soc. Rev. 2007, 36, 2070.
doi: 10.1039/b610738a |
|
|
(c) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, S. V. Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C |
|
|
(d) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
doi: 10.1021/cr4002879 |
|
|
(e) Wang, S.-W.; Yu, J.; Zhou, Q.-Y.; Chen, S.-Y.; Xu, Z.-H.; Tang, S. ACS Sustainable Chem. Eng. 2019, 7, 10154.
doi: 10.1021/acssuschemeng.9b02178 |
|
|
(f) Tang, S.; Yuan, L.; Deng, Y.-L.; Li, Z.-Z.; Wang, L.-N.; Huang, G.-X.; Sheng, R.-L. Tetrahedron Lett, 2017, 58, 329.
doi: 10.1016/j.tetlet.2016.12.027 |
|
| [2] |
(a) Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525.
doi: 10.1021/cr60274a001 |
|
(b) Yagupol’skii, L. M.; Il’chenko, A. Y.; Kondratenko, N. V. Russ. Chem. Rev. 1974, 43, 1.
doi: 10.1070/RC1974v043n01ABEH001785 |
|
|
(c) Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165.
doi: 10.1021/cr00002a004 |
|
|
(d) Leroux, F.; Jeschke, P.; Schlosser, M. Chem. Rev. 2005, 105, 827.
doi: 10.1021/cr040075b |
|
|
(e) Manteau, B.; Pazenok, S.; Vors, J. P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140.
doi: 10.1016/j.jfluchem.2009.09.009 |
|
|
(f) Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731.
doi: 10.1021/cr500193b |
|
|
(g) Ni, C.-F.; Hu, M.-Y. Hu, J.-B. Chem. Rev. 2015, 115, 765.
doi: 10.1021/cr5002386 |
|
|
(h) Shao, X.-X.; Xu, C.-F.; Lu, L.; Shen, Q.-L. Acc. Chem. Res. 2015, 48, 1227.
doi: 10.1021/acs.accounts.5b00047 |
|
|
(i) Chachignon, H.; Cahard, D. Chin J. Chem. 2016, 34, 445.
doi: 10.1002/cjoc.201500890 |
|
| [3] |
(a) Henkel, T.; Brunne, R. M.; Müller, H.; Reichel, F. Angew. Chem., Int. Ed. 1999, 38, 643.
doi: 10.1002/(SICI)1521-3773(19990301)38:5【-逻*辑*与-】#x00026;lt;643::AID-ANIE643【-逻*辑*与-】#x00026;gt;3.0.CO;2-G |
|
(b) Hili, R.; Yudin, A. K. Nat. Chem. Biol. 2006, 2, 284.
|
|
|
(c) Gui, Q.-W.; Wang, B.-B.; Zhu, S.; Li, F.-L.; Zhu, M.-X.; Yi, M.; Yu, J.-L.; Wu, Z.-L.; He, W.-M. Green Chem. 2021, 23, 4430.
doi: 10.1039/D1GC01017D |
|
| [4] |
McGrath, N. A.; Brichacek, M.; Njardarson, J. T. J. Chem. Educ. 2010, 87, 1348.
|
| [5] |
(a) Zhang, S.-B.; Xu, X.-H.; Qing, F.-L. J. Fluorine Chem. 2019, 227, 109367.
doi: 10.1016/j.jfluchem.2019.109367 |
|
(b) Liu, Y.-L.; Xu, X.-H.; Qing, F.-L. Adv. Synth. Catal. 2020, 362, 5031.
doi: 10.1002/adsc.202000861 |
|
| [6] |
(a) Xiang, H.-Y.; Yang, C.-H. Org. Lett. 2014, 16, 21, 5686.
|
|
(b) Gelat, F.; Poisson, T.; Biju, A.T.; Pannecoucke, X.; Besset, T. Eur. J. Org. Chem. 2018, 2018, 3693.
|
|
|
(c) Zhu, D.; Luo, H.-Y.; Chen, Z.-M. Org. Lett. 2021, 23, 1044.
doi: 10.1021/acs.orglett.0c04236 |
|
| [7] |
(a) Jin, D.-P.; Gao, P.; Gao, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2016, 18, 3486.
doi: 10.1021/acs.orglett.6b01702 |
|
(b) Modak, A.; Pinter, E. N.; Cook, S. P. J. Am. Chem. Soc. 2019, 141, 18405.
doi: 10.1021/jacs.9b10316 |
|
|
(c) Zheng, C.-G.; Huang, S.-A.; Liu, Y.; Jiang, C.; Zhang, W.; Fang, G.; Hong, J.-Q. Org. Lett. 2020, 22, 4868.
doi: 10.1021/acs.orglett.0c01714 |
|
|
(d) Yang, W.-C.; Zhang, M.-M.; Sun, Y.; Chen, C.-Y.; Wang, L. Org. Lett. 2021, 23, 6691.
doi: 10.1021/acs.orglett.1c02260 |
|
| [8] |
(a) Tang, S.; Deng, Y.-L.; Li, J.; Wang, W.-X.; Wang, Y.-C.; Li, Z.-Z.; Yuan, L.; Chen, S.-L.; Sheng, R.-L. Chem. Commun. 2016, 52, 4470.
doi: 10.1039/C5CC10464E |
|
(b) Yu, J.; Sheng, H.-X.; Wang, S.-W.; Xu, Z.-H.; Tang, S.; Chen, S.-L. Chem. Commun. 2019, 55, 4578.
doi: 10.1039/C9CC00294D |
|
|
(c) Zhang, G.; Liu, Y.; Zhao, J.-B.; Li, Y.; Zhang, Q. Sci. China Chem. 2019, 62, 1476.
doi: 10.1007/s11426-019-9629-x |
|
|
(c) Wu, X.-X.; Ma, Z.-G., Feng, T.-T.; Zhu, C. Chem. Soc. Rev. 2021, 50, 11577.
doi: 10.1039/D1CS00529D |
|
| [9] |
Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128.
doi: 10.1021/ol403739w |
| [10] |
Fuentes, N.; Kong, W.; Fernández-Sánchez, L.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 964.
doi: 10.1021/ja5115858 pmid: 25561161 |
| [11] |
Qiu, Y.-F.; Zhu, X.-Y.; Li, Y.-X.; He, Y.-T.; Yang, F.; Wang, J.; Hua, H.-L.; Zheng, L.; Wang, L.-C.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2015, 17, 3694.
doi: 10.1021/acs.orglett.5b01657 |
| [12] |
Guo, K.; Zhang, H.-L.; Cao, S.-J.; Gu, C.; Zhou, H.-T.; Li, J.; Zhu, Y.-G. Org. Lett. 2018, 20, 2261.
doi: 10.1021/acs.orglett.8b00614 |
| [13] |
Sun, K.; Lv, Q.-Y.; Lin, Y.-W.; Yu, B.; He, W.-M. Org. Chem. Front. 2021, 8, 445.
doi: 10.1039/D0QO01058H |
| [14] |
Fu, H.; Wang, S.-S.; Li, Y.-M. Adv. Synth. Catal. 2016, 358, 3616.
doi: 10.1002/adsc.201600693 |
| [15] |
(a) Sun, K.; Wang, X.; Li, C.; Wang, H.; Li, L. Org. Chem. Front. 2020, 7, 3100.
doi: 10.1039/D0QO00849D |
|
(b) Sun, K.; Li, Y.-L.; Feng, R.-R.; Mu, S.-Q.; Wang, X.; Zhang, B. J. Org. Chem. 2020, 85, 1001.
doi: 10.1021/acs.joc.9b02941 |
|
|
(c) Sun, K.; Lei, J.; Liu, Y.; Liu, B.; Chen, N. Adv. Synth. Catal. 2020, 362, 3709.
doi: 10.1002/adsc.202000876 |
|
|
(d) Wang, X.; Guo, S.; Zhang, Y.; Zhang, Z.; Zhang, G.; Ye, Y.; Sun, K. Adv. Synth. Catal. 2021, 363, 3290.
doi: 10.1002/adsc.202100208 |
|
|
(e) Wang, X.; Lei, J.; Guo, S.; Zhang, Y.; Ye, Y.; Tang, S.; Sun, K. Chem. Commun. 2022, 58, 1526.
doi: 10.1039/D1CC06323E |
|
|
(f) Zhang, Z.; Wang, S.-L.; Tan, P.-P.; Gu, X.-W.; Sun, W.-J.; Liu, C.; Chen, J.-C.; Li, J.-Z.; Sun, K. Org. Lett. 2022, 24, 12, 2288.
|
|
| [16] |
(a) Hu, M.; Fan, J.-H.; Liu, Y.; Ouyang, X.-H.; Song, R.-J.; Li, J.-H. Angew. Chem., Int. Ed. 2015, 54, 9577.
doi: 10.1002/anie.201504603 |
|
(b) Wang, A.-F.; Zhu, L.-Y.; Wang, S.-L.; Hao, W.-J.; Li, G.-G.; Tu, S.-J.; Jiang, B. J. Org. Chem. 2016, 81, 1099.
doi: 10.1021/acs.joc.5b02655 |
| [1] | 高宝昌, 石雨, 田媛, 张治国, 张婧如, 孙宇峰, 毛国梁, 戴凌燕. 4-甲基-2-氧代-6-芳氨基-二氢-吡喃-3-腈衍生物的合成[J]. 有机化学, 2024, 44(2): 644-649. |
| [2] | 夏登鹏, 罗锦昀, 何林, 蔡志华, 杜广芬. 氮杂环卡宾催化的五氟苯基硫醚的合成[J]. 有机化学, 2024, 44(2): 622-630. |
| [3] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [4] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [5] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [6] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [7] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [8] | 蔡远林, 吕亚, 聂桂花, 金智超, 池永贵. 氮杂环卡宾催化合成氰基化合物的研究进展[J]. 有机化学, 2023, 43(9): 3135-3145. |
| [9] | 张建涛, 张聪, 莫诺琳, 罗佳婷, 陈莲芬, 刘卫兵. 氯仿参与的烯烃自由基加成反应的研究进展[J]. 有机化学, 2023, 43(9): 3098-3106. |
| [10] | 陈祖良, 魏颖静, 张俊良. 供体-受体氮杂环丙烷碳-碳键断裂的环加成反应研究进展[J]. 有机化学, 2023, 43(9): 3078-3088. |
| [11] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [12] | 徐伟, 翟宏斌, 程斌, 汪太民. 可见光诱导的钯催化Heck反应[J]. 有机化学, 2023, 43(9): 3035-3054. |
| [13] | 樊思捷, 董武恒, 梁彩云, 王贵超, 袁瑶, 尹作栋, 张兆国. 可见光诱导的自由基环化反应构建4-芳基-1,2-二氢萘类化合物[J]. 有机化学, 2023, 43(9): 3277-3286. |
| [14] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [15] | 赵瑜, 张凯, 白育斌, 张琰图, 史时辉. 无金属条件下可见光催化与溴盐协同促进烯烃的氢硅化反应研究[J]. 有机化学, 2023, 43(8): 2837-2847. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
