有机化学 ›› 2024, Vol. 44 ›› Issue (4): 1160-1180.DOI: 10.6023/cjoc202310009 上一篇 下一篇
综述与进展
彭天凤a, 赵玉祥b, 浦绍健a, 罗娟b, 刘腾b,*( ), 缪应纯b,*(
), 缪应纯b,*( ), 沈先福b,*(
), 沈先福b,*( )
)
收稿日期:2023-10-08
修回日期:2023-11-10
发布日期:2023-11-23
基金资助:
Tianfeng Penga, Yuxiang Zhaob, Shaojian Pua, Juan Luob, Teng Liub( ), Yingchun Miaob(
), Yingchun Miaob( ), Xianfu Shenb(
), Xianfu Shenb( )
)
Received:2023-10-08
Revised:2023-11-10
Published:2023-11-23
Contact:
E-mail: Supported by:文章分享

异戊烯基吲哚生物碱是一类结构有趣和重要的天然产物家族化合物, 其典型结构特征是有一个吲哚环或其衍生物(即螺环氧化吲哚或假吲哚), 由一个或多个异戊烯基或异戊烯基的残基修饰. 异戊烯基吲哚生物碱组成了一个庞大和结构多样化的具有广泛且重要的生物活性天然产物家族化合物. 自然界以焦磷酸二甲基烯丙基(DMAPP)和焦磷酸异戊烯基(IPP)为起始原料, 生物合成了许多异戊烯基吲哚生物碱, 而异戊烯基吲哚生物碱的吲哚核心通常来源于L-色氨酸, 或者少部分来源于吲哚-3-甘油磷酸酯. 异戊烯基化是几乎所有生物普遍存在的过程, 是有机合成中的关键转化. 近年来, 异戊烯基吲哚生物碱因其独特的结构单元和多样的生物学活性, 受到合成化学家和药理学家的持续深入研究. 迄今为止, 发展一类直接和高效的方法来合成结构多样的异戊烯基吲哚生物碱是非常理想的, 同时也是非常具有挑战性的研究工作. 综述了近期过渡金属催化的关键反应在异戊基化吲哚生物碱全合成中的研究进展.
彭天凤, 赵玉祥, 浦绍健, 罗娟, 刘腾, 缪应纯, 沈先福. 过渡金属催化的关键反应在异戊烯基吲哚生物碱全合成中的研究进展[J]. 有机化学, 2024, 44(4): 1160-1180.
Tianfeng Peng, Yuxiang Zhao, Shaojian Pu, Juan Luo, Teng Liu, Yingchun Miao, Xianfu Shen. Recent Advances in Total Synthesis of Prenylated Indole Alkaloids by Transition Metal-Catalyzed Reactions as the Key Step[J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1160-1180.
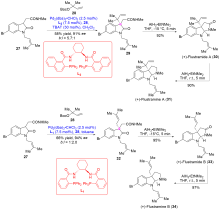

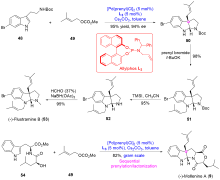

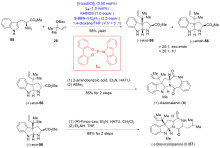



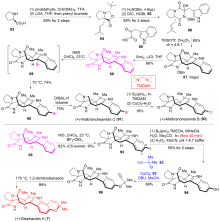

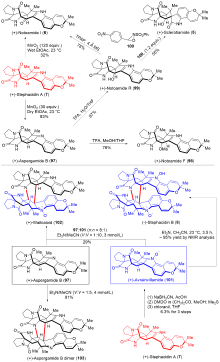

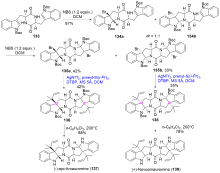

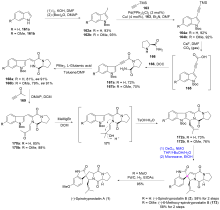



| [1] |
Lindel T.; Marsch N.; Adla S. K. Top. Curr. Chem. 2011, 309, 67.
|
| [2] |
Li S.-M. Nat. Prod. Rep. 2010, 27, 57.
doi: 10.1039/B909987P |
| [3] |
Williams R. M.; Stocking E. M.; Sanz-Cervera J. F. Top. Curr. Chem. 2000, 209, 97.
|
| [4] |
Tanner M. E. Nat. Prod. Rep. 2015, 32, 88.
doi: 10.1039/C4NP00099D |
| [5] |
Miller K. A.; Williams R. B. Chem. Soc. Rev. 2009, 38, 3160.
doi: 10.1039/b816705m |
| [6] |
Walsh C. T.; Garneau-Tsodikova S.; Gatto G. J. Jr. Angew. Chem., Int. Ed. 2005, 44, 7342.
doi: 10.1002/anie.v44:45 |
| [7] |
Palsuledesai C. C.; Distefano M. D. ACS Chem. Biol. 2015, 10, 51.
doi: 10.1021/cb500791f pmid: 25402849 |
| [8] |
Jeong A.; Auger S. A.; Maity S.; Fredriksen K.; Zhong R.; Li L.; Distefano M. D. ACS Chem. Biol. 2022, 17, 2863.
doi: 10.1021/acschembio.2c00486 |
| [9] |
Klas K. R.; Kato H.; Frisvad J. C.; Yu F. G.; Newmister S. A.; Fraley A. E.; Sherman D. H.; Tsukamoto S.; Williams R. M. Nat. Prod. Rep. 2018, 35, 532.
doi: 10.1039/C7NP00042A |
| [10] |
Ishikura M.; Abe T.; Choshi T.; Hibino S. Nat. Prod. Rep. 2013, 30, 694.
doi: 10.1039/c3np20118j pmid: 23467716 |
| [11] |
Ishikura M.; Abe T.; Choshi T.; Hibino S. Nat. Prod. Rep. 2015, 32, 1389.
doi: 10.1039/c5np00032g pmid: 26151910 |
| [12] |
Oldfield E.; Lin F.-Y. Angew. Chem., Int. Ed. 2012, 51, 1124.
doi: 10.1002/anie.201103110 pmid: 22105807 |
| [13] |
Sacchettini J. C.; Poulter C. D. Science 1997, 277, 1788.
doi: 10.1126/science.277.5333.1788 pmid: 9324768 |
| [14] |
Walsh C. T.; Garneau-Tsodikova S.; Gatto G. J. Jr. Angew. Chem., Int. Ed. 2005, 44, 7342.
doi: 10.1002/anie.v44:45 |
| [15] |
Chang W.-C.; Song H.; Liu H.-W.; Liu P. Curr. Opin. Chem. Biol. 2013, 17, 571.
doi: 10.1016/j.cbpa.2013.06.020 |
| [16] |
Hu Y.-C.; Ji D.-W.; Zhao C.-Y.; Zheng H.; Chen Q.-A. Angew. Chem., Int. Ed. 2019, 58, 5438.
doi: 10.1002/anie.v58.16 |
| [17] |
Zhao C.-Y.; Liu Y.-Y.; Zhang X.-X.; He G.-C.; Liu H.; Ji D.-W.; Hu Y.-C.; Chen Q.-A. Angew. Chem., Int. Ed. 2022, 61, e202207202.
doi: 10.1002/anie.v61.32 |
| [18] |
Chang X. X.; Zhang F. Q.; Zhu S. B.; Yang Z.; Feng X. M.; Liu Y. B. Nat. Commun. 2023, 14, 3876.
doi: 10.1038/s41467-023-39633-9 |
| [19] |
Zhang G.; Zhao C.-Y.; Min X.-T.; Li Y.; Zhang X.-X.; Liu H.; Ji D.-W.; Hu Y.-C.; Chen Q.-A. Nat. Catal. 2022, 5, 708.
doi: 10.1038/s41929-022-00825-z |
| [20] |
Itoh J.; Han S. B.; Krische M. J. Angew. Chem., Int. Ed. 2009, 48, 6313.
doi: 10.1002/anie.v48:34 |
| [21] |
Kimura M.; Futamata M.; Mukai R.; Tamaru Y. J. Am. Chem. Soc. 2005, 127, 4592.
doi: 10.1021/ja0501161 |
| [22] |
Usui I.; Schmidt S.; Keller M.; Breit B. Org. Lett. 2008, 10, 1207.
doi: 10.1021/ol800073v |
| [23] |
Sundararaju B.; Achard M.; Demerseman B.; Toupet L.; Sharma G. V. M.; Bruneau C. Angew. Chem., Int. Ed. 2010, 49, 2782.
doi: 10.1002/anie.200907034 pmid: 20229550 |
| [24] |
Yang H. W.; Fang L.; Zhang M.; Zhu C. J. Eur. J. Org. Chem. 2009, 5, 666.
|
| [25] |
Hu Y.-C.; Ji D.-W.; Zhao C.-Y.; Zheng H.; Chen Q.-A. Angew. Chem., Int. Ed. 2019, 58, 5438.
doi: 10.1002/anie.v58.16 |
| [26] |
Trost B. M.; Stilels D. T. Org. Lett. 2008, 10, 3701.
doi: 10.1021/ol8013073 |
| [27] |
Trost B. M.; Malhotra S.; Chan W. H. J. Am. Chem. Soc. 2011, 133, 7328.
doi: 10.1021/ja2020873 |
| [28] |
Kitahara K.; Shimokawa J.; Fukuyama T. Chem. Sci. 2014, 5, 904.
doi: 10.1039/C3SC52525B |
| [29] |
Tu H.-F.; Zhang X.; Zheng C.; Zhu M.; You S.-L. Nat. Catal. 2018, 1, 601.
doi: 10.1038/s41929-018-0111-8 |
| [30] |
Ruchti J.; Carreira E. M. J. Am. Chem. Soc. 2014, 136, 16756.
doi: 10.1021/ja509893s |
| [31] |
Müller J. M.; Stark C. B. W.; Angew. Chem., Int. Ed. 2016, 55, 4798.
doi: 10.1002/anie.201509468 pmid: 26969898 |
| [32] |
Liang X.; Zhang T.-Y; Zeng X.-Y.; Zheng Y.; Wei K.; Yang Y.-R. J. Am. Chem. Soc. 2017, 139, 3364.
doi: 10.1021/jacs.7b00854 pmid: 28219006 |
| [33] |
Feng Y.; Holte D.; Zoller J.; Umemiya S.; Simke L. R.; Baran P. S. J. Am. Chem. Soc. 2015, 137, 10160.
doi: 10.1021/jacs.5b07154 pmid: 26256033 |
| [34] |
Mukai K.; de Sant’Ana D. P.; Hirooka Y.; Mercado-Marrin E. V.; Stephens D. E.; Kou K. G. M.; Richter S. C.; Kelley N.; Sarpong R. Nat. Chem. 2018, 10, 38.
doi: 10.1038/nchem.2862 pmid: 29256515 |
| [35] |
Frebault F. C.; Simpkins N. S. Tetrahedron 2010, 66, 6585.
doi: 10.1016/j.tet.2010.04.093 |
| [36] |
Peng T. F.; Liu T.; Zhao J. F.; Dong J. W.; Zhao Y. X.; Yang Y. X.; Yan X.; Xu W. L.; Shen X. F. J. Org. Chem. 2022, 83, 16743.
|
| [37] |
Song H. Q.; Song J. C.; Yan L. H.; He W. G.; Wang P. Y.; Xu Y. Z.; Wei H. B.; Xie W. Q. Tetrahedron Lett. 2021, 85, 153486.
doi: 10.1016/j.tetlet.2021.153486 |
| [38] |
Wang Y.; Kong C.; Du Y.; Song H.; Zhang D.; Qin Y. Org. Biomol. Chem. 2012, 10, 2793.
doi: 10.1039/c2ob00014h pmid: 22383065 |
| [39] |
Hakamata H.; Sato S.; Ueda H.; Tokuyama H. Org. Lett. 2017, 19, 5308.
doi: 10.1021/acs.orglett.7b02602 |
| [40] |
Chen Z. G.; Zhong W.; Liu S. H.; Zou T.; Zhang K. Q.; Gong C. L.; Guo W. Y.; Kong F. Z.; Nie L. B.; Hu S. Q.; Wang H. F. Org. Lett. 2023, 25, 3391.
doi: 10.1021/acs.orglett.3c00904 |
| [41] |
Yang J.; Singh B.; Cohen G.; Ting C. P. J. Am. Chem. Soc. 2023, 145, 19189.
doi: 10.1021/jacs.3c07078 |
| [42] |
Xi Y.-K.; Zhang H. B.; Li R.-X.; Kang S.-Y.; Li J.; L Y. Chem.-Eur. J. 2019, 25, 3005.
doi: 10.1002/chem.v25.12 |
| [43] |
Zhang B. X.; Zheng W. F.; Wang X. Q.; Sun D. Q.; Li C. Z. Angew. Chem., Int. Ed. 2016, 55, 1.
|
| [44] |
Hou Y.; Huo J. Y.; Li R. X.; Hou J.; Lei P.; Wei H. B.; Xie W. Q. Org. Lett. 2023, 25, 6949.
doi: 10.1021/acs.orglett.3c02296 pmid: 37713279 |
| [1] | 鞠国栋, 周冠宇, 赵应声. 三异丙基硅烷(TIPS)保护苯酚的无过渡金属催化区域选择性硫氰化反应[J]. 有机化学, 2024, 44(4): 1327-1336. |
| [2] | 万云辉, 杨福美, 陈明瀚, 孙德立, 叶丹锋. 无过渡金属催化的N-苄基-N-叔丁氧羰基酰胺与不饱和醇的酯化反应[J]. 有机化学, 2024, 44(4): 1293-1300. |
| [3] | 李晨龙, 余志祥. 一氧化碳参与的过渡金属催化的插羰环加成反应研究进展[J]. 有机化学, 2024, 44(4): 1045-1068. |
| [4] | 郭凯杰, 符昕姝, 李靖, 陈艳, 胡美丽, 堵锡华, 谢屿阳, 何燕. 过渡金属催化C—S键活化与转化研究进展[J]. 有机化学, 2024, 44(4): 1124-1150. |
| [5] | 高淳, 刘欣, 王明慧, 刘淑贤, 朱婷婷, 张怡康, 郝二军, 杨启亮. 电化学不对称合成反应的研究进展[J]. 有机化学, 2024, 44(3): 673-727. |
| [6] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [7] | 高晓阳, 翟锐锐, 陈训, 王烁今. 碳酸亚乙烯酯参与C—H键活化反应的研究进展[J]. 有机化学, 2023, 43(9): 3119-3134. |
| [8] | 丁卫忠, 张炳文, 薛彦青, 林雨琦, 汤志军, 王婧, 杨文超, 王晓峰, 刘文. 禾谷镰刀菌中一个新的聚酮类化合物[J]. 有机化学, 2023, 43(9): 3319-3322. |
| [9] | 吴秀蓉, 肖朝江, 沈怡, 汤红霞, 朱俊逸, 姜北. 植物来源抗疟倍半萜类天然产物研究(1972~2022)[J]. 有机化学, 2023, 43(8): 2764-2789. |
| [10] | 陈新强, 张敬. 伯醇的脱羟甲基反应的研究进展[J]. 有机化学, 2023, 43(8): 2711-2719. |
| [11] | 户晓兢, 郭斐翔, 朱润青, 周柄棋, 张涛, 房立真. 对烷氧基酚的合成及其去芳构化后的合成应用[J]. 有机化学, 2023, 43(6): 2239-2244. |
| [12] | 徐光利, 许静, 徐海东, 崔香, 舒兴中. 过渡金属催化烯烃和炔烃合成1,3-共轭二烯化合物研究进展[J]. 有机化学, 2023, 43(6): 1899-1933. |
| [13] | 刘兴周, 于明加, 梁建华. 原小檗碱骨架的合成及其抗炎活性研究进展[J]. 有机化学, 2023, 43(4): 1325-1340. |
| [14] | 庞明杨, 常宏宏, 冯璋, 张娟. 过渡金属催化吲哚的串联去芳构化反应研究进展[J]. 有机化学, 2023, 43(4): 1271-1291. |
| [15] | 贾海瑞, 邱早早. 过渡金属催化硼-氢键活化合成含硼-杂原子键邻碳硼烷衍生物的研究进展[J]. 有机化学, 2023, 43(3): 1045-1068. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||