研究论文
侯馨怡a, 史同同b, 李泽江a,*, 董庆昊b, 孙凯b,*, 王薪b,*
收稿日期:2025-09-25
修回日期:2025-10-20
基金资助:Xinyi Houa, Tongtong Shib, Zejiang Lia,*, Qinghao Dongb, Kai Sunb,*, Xin Wangb,*
Received:2025-09-25
Revised:2025-10-20
Contact:
*E-mail: Supported by:文章分享
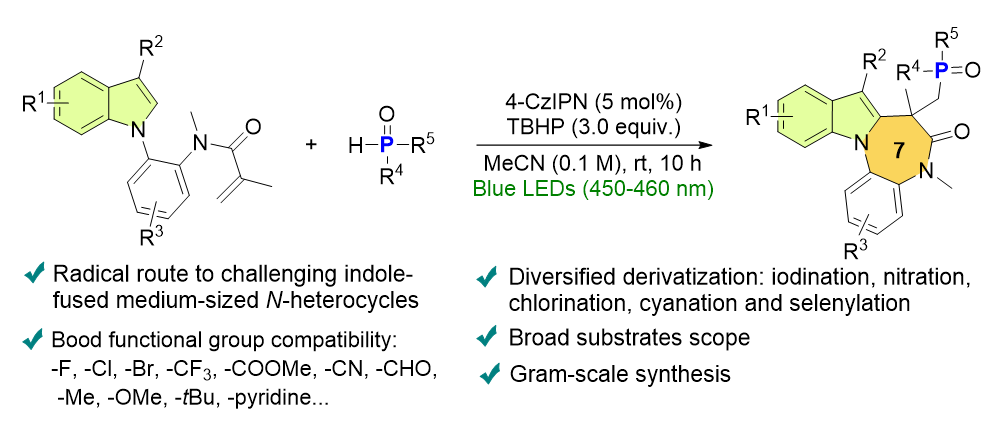
在室温下,本工作发展了一种新颖的可见光诱导的磷酰化环化合成磷酰基取代的吲哚并二氮草。广泛的底物范围,良好的官能团兼容性,放大量合成,以及通过硝化,氯化和氰基化等衍生化反应证明了该方案的实用性。通过自由基抑制实验、开关光照射实验、AQE计算、紫外-可见吸收光谱研究和循环伏安实验,提出了能量转移介导的磷中心自由基反应途径。
侯馨怡, 史同同, 李泽江, 董庆昊, 孙凯, 王薪. 可见光催化的磷酰化环化合成磷酰基取代的吲哚并二氮草[J]. 有机化学, doi: 10.6023/cjoc202509032.
Xinyi Hou, Tongtong Shi, Zejiang Li, Qinghao Dong, Kai Sun, Xin Wang. Visible-Light-Induced Phosphorylation Cyclization to Phosphorylated Indole-Fused Diazepines[J]. Chinese Journal of Organic Chemistry, doi: 10.6023/cjoc202509032.
| [1] Li J. L.; Zhao Q.; Gou C.; Li Q. Z.; Leng H. J.; Huang Q. W.; Liu, Y. Construction of Indole-Fused Heterocycles Starting from 2-Thioxoindolines, Iminoindolines, and Their Derivatives. Adv. Synth. Catal.2021, 363, 4497-4515. [2] (a) Mizoguchi H.; Oikawa H.; Oguri H. Biogenetically Inspired Synthesis and Skeletal Diversification of Indole Alkaloids. Nat. Chem.2014, 6, 57-64 (b) Purgatorio R.; Candia M. de.; Catto M.; Carrieri A.; Pisani L.; Palma A. De.; Toma M.; Ivanova O. A.; Voskressensky L. G.; Altomare, C. D. Investigating 1,2,3,4,5,6-Hexahydroazepino[4,3-b]indole as Scaffold of Butyrylcholinesterase-Selective Inhibitors with Additional Neuroprotective Activities for Alzheimer's Disease. Eur. J. Med. Chem.2019, 177, 414-424. [3] Faust R.; Garratt P. J.; Jones R.; Yeh L. K.; Tsotinis A.; Panoussopoulou M.; Calogeropoulou T.; The M. T.; Sugden D. Mapping the Melatonin Receptor. 6. Melatonin Agonists and Antagonists Derived from 6H-Isoindolo[2,1-a]indoles, 5,6-Dihydroindolo[2,1-a]isoquinolines, and 6,7-Dihydro-5H-Benzo[c]azepino[2 1-a]indoles. J. Med. Chem.2000, 43, 1050-1061. [4] (a) Disney A. J. M.; Kellam B.; Dekker, L. V. Alkylation of Staurosporine to Derive a Kinase Probe for Fluorescence Applications. ChemMedChem.2016, 11, 972-979 (b) Gayler K. M.; Kong K.; Reisenauer K.; Taube J. H.; Wood, J. L. Staurosporine Analogs via C-H Borylation. ACS Med. Chem. Lett.2020, 11, 2441-2445. [5] (a) Ohno H.; Hamaguchi H.; Ohata M.; Tanaka, T. Bromoallenes as Synthetic Equivalents of Allyl Dications: Synthesis of Medium-Sized Nitrogen Heterocycles Through the Cyclization of Bromoallenes in the Presence of a Palladium(0) Catalyst and an Alcohol. Angew. Chem. Int. Ed.2003, 42, 1749-1753 (b) Shiina, I. Total Synthesis of Natural 8- and 9-Membered Lactones:? Recent Advancements in Medium-Sized Ring Formation. Chem. Rev.2007, 107, 239-273. [6] (a) Lian G.; Li J.; Liu P.; Sun, P. Photoredox-Catalyzed Radical Cascade Reaction to Synthesize Fluorinated Pyrrolo[1,2-d]benzodiazepine Derivatives. J. Org. Chem.2019, 84, 9322-9329 (b) Liu Y. W.; Wang M. M.; Zhang Y. Q.; Xu H.; Dai, H. X. Construction of Indole-Fused Seven- and Eight-Membered Azaheterocycles via a Tandem Pd/NBE-Catalyzed Decarbonylation and Dual C-H Activation Sequence. Org. Lett.2023, 25, 5406-5410 (c) Chiu W. J.; Chu T. Y.; Barve I. J.; Sun C. M. Pd(II)-Catalyzed [5 + 2] Cyclization of N-Triflyl Aryl Indoles and α,γ-Substituted Allenoates: A Route to Indole-Fused Benzodiazepines. Org. Lett.2023, 25, 6246-6250 (d) Guo L.; Zhang Z.; Zhang F.; Sun K.; Yu, B. Visible-Light-Induced Cascade Cyclization of 1-(2-(Arylethynyl)benzoyl)indoles into Sulfonated Benazepino[1,2-a]indolones. Org. Lett.2024, 26, 10982-10987 (e) Li J.; Ni H.; Zhang W.; Lai Z.; Jin H.; Zeng L.; Cui, S. A Multicomponent Reaction for Modular Assembly of Indole-Fused Heterocycles. Chem. Sci.2024, 15, 5211. [7] (a) Hua H. L.; Zhang B. S.; He Y. T.; Qiu Y. F.; Hu J. Y.; Yang Y. C.; Liang, Y. M. Copper-Catalyzed Difluoromethylation of Propargylamide-Substituted Indoles: Synthesis of Mono- and Bis-Difluoromethylated Indoloazepinone Derivatives. Chem. Commun.2016, 52, 10396-10399 (b) Zhao L.; Yan Z. H.; Tang S.; Wei Z. L.; Liao, W. W. Brønsted Acid-Promoted Cyclodimerization of Indolyl Ketones: Construction of Indole Fused-Oxabicyclo[3.3.1]nonane and -Cyclooctatetraene Ring Systems. Org. Lett.2020, 23, 166-171 (c) Huang X.; Shi Y.; Wang Y.; Jiao J.; Tang Y.; Li J.; Xu S.; Li, Y. Synthesis of Indole-Fused Oxepines via C-H Activation Initiated Diastereoselective [5 + 2] Annulation of Indoles with 1,6-Enynes. Org. Lett.2021, 23, 8365-8369 (d) Qiu Z. W.; Li B. Q.; Liu H. F.; Zhu Z. Q.; Pan H. P.; Feng N.; Ma A. J.; Peng J. B.; Zhang X. Z. Formal (3 + 4)-Annulation of Propargylic p-Quinone Methides with 2-Indolylmethanols: Synthesis of Polysubstituted Indole-Fused Oxepines. J. Org. Chem.2021, 86, 7490-7499 (e) Jin H. S.; Fang Q. Y.; Wang J. Q.; Zhao, L. M. Divergent Reaction of Indoline-derived Azadienes with α-Bromohydroxamates: Synthesis of Spiro-Indolinepyrrolidinones and Indoline-Fused Diazepinones. Chem. Eur. J.2023, 29, e202300467 (f) Chen L. Q.; Zhu C. F.; Zhang S.; Liu B. Y.; Tu S. J.; Hao W. J.; Jiang, B. Palladium-Catalyzed Annulative Allylic Alkylation for Regioselective Construction of Indole-Fused Medium-Sized Cyclic Ethers. Chin. Chem. Lett.2023, 34, 108398 (g) Zhang J. Y.; Chen J. Y.; Gao C. H.; Yu L.; Ni S. F.; Tan W.; Shi f. Asymmetric (4+n) Cycloadditions of Indolyldimethanols for the Synthesis of Enantioenriched Indole-Fused Rings. Angew. Chem. Int. Ed.2023, 62, e202305450. [8] (a) Harada S.; Yanagawa M.; Nemoto, T. Dual-Functional Enone-Directing Group/Electrophile for Sequential C-C Bond Formation with α-Diazomalonates: A Short Synthesis of Chiral 3,4-Fused Tricyclic Indoles. ACS Catal.2020, 10, 11971-11979 (b) Chen C.; Zuo X.; Tu D.; Wan B.; Zhang, Y. Synthesis of 3,4-Fused Tricyclic Indoles through Cascade Carbopalladation and C-H Amination: Development and Total Synthesis of Rucaparib. Org. Lett.2020, 22, 4985-4989 (c) Yang S.; An X. D.; Qiu B.; Liu R. B.; Xiao, J. Access to Polycyclic Indole-3,4-Fused Nine-Membered Ring via Cascade 1,6-Hydride Transfer/Cyclization. Org. Lett.2021, 23, 9100-9105 (d) Antropov S. M.; Tokmacheva S. A.; Levina I. I.; Ivanova O. A.; Trushkov, I. V. Synthesis of Bridged Bicyclic Systems peri-Annulated to the Indole Ring: Tropane-Fused Indoles. Adv. Synth. Catal.2024, 366, 2784-2490. [9] Rapelli C.; Sridhar B.; Reddy, B. V. S. Tandem Prins Cyclization for the Synthesis of Indole Fused Spiro-1,4-Diazocane Scaffolds. Org. Biomol. Chem.2020, 18, 6710-6715. [10] (a) Staveness D.; Bosque I.; Stephenson, C. R. Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer. Acc. Chem. Res.2016, 49, 2295 (b) Woźniak Ł.; Magagnano G.; Melchiorre, P. Enantioselective Photochemical Organocascade Catalysis. Angew. Chem. Int. Ed.2018, 57, 1068 (c) Staveness D.; Collins III J. L.; McAtee, R. C. Stephenson, C. R. Exploiting Imine Photochemistry for Masked N-Centered Radical Reactivity. Angew. Chem. Int. Ed.2019, 58, 19000 (d) Latrache M.; Hoffmann, N. Photochemical Radical Cyclization Reactions with Imines, Hydrazones, Oximes and Related Compounds. Chem. Soc. Rev.2021, 50, 7418 (e) Lu M.-J.; Liang R.-B.; Zhu C.-M.; Tong Q.-X.; Zhong, J,-J. Chin. J. Chem.2023, 41, 1823 (f) Liang R.-B.; Miao T.-T.; Li X.-R.; Huang J.-B.; Ni S.-F.; Li S.-L.; Tong Q.-X.; Zhong, J -J. Chem. Sci.2025, 16, 3580 (g) Zhang R.-J.; Li, X.-R. Liang R.-B.; Xiao Y.-H.; Tong Q.-X.; Zhong, J -J.; Wu L.-Z. Org. Lett.2024, 26, 591. [11] (a) Wille, U. Radical Cascades Initiated by Intermolecular Radical Addition to Alkynes and Related Triple Bond Systems. Chem. Rev.2013, 113, 813 (b) Zhang B.; Studer, A. Recent Advances in the Synthesis of Nitrogen Heterocycles via Radical Cascade Reactions Using Isonitriles as Radical Acceptors. Chem. Soc. Rev.2015, 44, 3505 (c) Chen Z. M.; Zhang X. M.; Tu Y. Q. Radical Aryl Migration Reactions and Synthetic Applications. Chem. Soc. Rev.2015, 44, 5220 (d) Stuyver T.; Chen B.; Zeng T.; Geerlings P.; Proft F. D.; Hoffmann, R. Do Diradicals Behave Like Radicals. Chem. Rev.2019, 119, 11291 (e) Wu, X.; Ma, Z.; Feng, T.; Zhu, C. Radical-Mediated Rearrangements: Past, Present, and Future. Chem. Soc. Rev. 2021, 50, 11577; (f) Coppola G. A.; Pillitteri S.; Van der Eycken, E. V.; You S. L.; Sharma U. K. Multicomponent Reactions and Photo/Electrochemistry Join Forces: Atom Economy Meets Energy Efficiency. Chem. Soc. Rev.2022, 51, 2313. [12] (a) Baumgartner T.; Réau, R. Organophosphorus π-Conjugated Materials. Chem. Rev.2006, 106, 4681-4727 (b) De Clercq, E. The Next Ten Stories on Antiviral Drug Discovery (part E): Advents, Advances, and Adventures. Med. Res. Rev. 2011, 31, 118-160; (c) Queffélec, C.; Petit, M.; Janvier, P.; Knight, D. A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777-3807. [13] (a) Cai B. G.; Xuan J.; Xiao, W. J. Visible Light-Mediated C-P Bond Formation Reactions. Sci. Bull.2019, 64, 337 (b) Zeng F. L.; Jia Z.; Loh, T. P. Recent Advances in Visible-Light-Mediated Synthesis of Phosphorylated Heterocycles. Adv. Synth. Catal.2024, 366, 4536-4547. [14] (a) Zhang Z.; Tan P.; Wang S.; Wang H.; Xie L.; Chen Y.; Han L.; Yang S.; Sun, K. Visible-Light-Promoted Selective Sulfonylation and Selenylation of Dienes to Access Sulfonyl-/Seleno-Benzazepine Derivatives. Org. Lett.2023, 25, 4208-4213 (b) Zhang Z.; Fang X.; Aili A.; Wang S.; Tang J.; Lin W.; Xie L.; Chen J.; Sun, K. Cascade Radical Trifluoromethylthiolation/Cyclization of Dienes to Access SCF3-Containing Medium-Sized Heterocycles. Org. Lett.2023, 25, 4598-4602 (c) Sun K.; Zhao D.; Li Q.; Ni S.; Zheng, G. Zhang, Q. Synthesis of Medium-Sized Benzo[b]azocines and Benzo[b]azonines by Photoinduced 8-/9-endo Sulfonyl-Cyclization. Sci. China Chem.2023, 66, 2309-2316 (d) Yin Y.-F.; Liu F.; Tian M.; Han L.-L.; Li M.-H.; Tao J.-F.; liu Q.; Sun L.-L.; Xu X.-M.; Sun, K. J. Org. Chem.2025, 90, 7070. [15] Zhao D.Y.; Wang X.; Huang J.-B.; Yu T.-T.; Hao E.-J.; Ni S.-F.; Sun K.Org. Lett. 2025, doi: 10.1021/acs.orglett.4c04632. [16] (a) Luo, J.; Zhang, J. Donor-Acceptor Fluorophores for Visible-Light-Promoted Organic Synthesis: Photoredox/Ni Dual Catalytic C(sp3)-C(sp2) Cross-Coupling. ACS Catal. 2016, 6, 873-877; (b) Shang T. Y.; Lu L. H.; Cao Z.; Liu Y.; He W. M.; Yu, B. Recent Advances of 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-Dicyanobenzene (4CzIPN) in Photocatalytic Transformations. Chem. Commun.2019, 55, 5408-5419. [17] (a) Zeng F. L.; Zhang Z. Y.; Yin P. C.; Cheng F. K.; Chen X. L.; Qu L. B.; Cao Z. Y.; Yu, B. Visible-Light-Induced Cascade Cyclization of 3-(2-(Ethynyl)phenyl)quinazolinones to Phosphorylated Quinolino[2,1-b]quinazolinones. Org. Lett.2022, 24, 7912-7917 (b) Lu Y. H.; Wu C.; Hou J. C.; Wu Z. L.; Zhou M. H.; Huang X. J.; He, W. M. Ferrocene-Mediated PhotoCatalytic Annulation of N-Sulfonyl Ketimines on a Polycrystalline WSe2 Semiconductor Photocatalyst. ACS Catal.2023, 13, 13071-13076 (c) Huang Q.; Liu J.; Wan, J. P Electrochemical Enaminone-Thioamide Annulation and Thioamide Dimeric Annulation for the Tuna-Ble Synthesis of Thiazoles and 1,2 4-Thiadiazole. Org. Lett.2024, 26, 5263-5268 (d) Zhou Y.; Yang W. H.; Dai N. N.; Feng J. Y.; Yang M. Q.; Gao W.; Li, Q. Deng, C. Lu, Z. Wei, W. T. Dual Nickel/photoredox-Catalyzed Arylsulfonylation of Allenes. Org. Lett.2024, 26, 5074-5081 (e) Lv Y.; Ding H.; You J.; Wei W.; Yi, D. Addi-Tive-Free Synthesis of S-Substituted Isothioureas via Visible-Light-Induced Four-Component Reaction of α-Diazoesters, Aryl Isothiocya-nates, Amines and Cyclic Ethers. Chin. Chem. Lett.2024, 35, 109107. |
| [1] | 祝辉, 吴鹏, 钟晨鸣, 李舒铭, 林钢, 刘雪粉, 罗书平. 供体-受体-供体(D-A-D)型芳香酮设计合成与光催化C(sp3)—H偶联反应性能研究[J]. 有机化学, 2025, 45(9): 3441-3449. |
| [2] | 解人杰, 谢复开, 孙然, 王欣, 王钰佳, 李蕾, 王贺. 电子供体-受体复合物(EDA)介导N-芳基丙烯酰胺与芳基硫鎓盐的自由基环化反应[J]. 有机化学, 2025, 45(8): 2913-2922. |
| [3] | 史茜, 李忠玉, 李晗. 杂环金属铱配合物光敏剂的研究进展[J]. 有机化学, 2025, 45(7): 2389-2405. |
| [4] | 谭永波, 舒洪波, 黄华文. 光诱导N-芳基丙烯酰胺参与的吲哚酮合成研究进展[J]. 有机化学, 2025, 45(6): 2086-2108. |
| [5] | 高根伟, 李震, 李炎, 陆熹. 光/镍协同催化C(sp2)—C(sp3)键构建研究进展[J]. 有机化学, 2025, 45(6): 1905-1919. |
| [6] | 李顺曦, 游力栩, 李玉龙, 舒伟. 光介导氨及其等价体参与的碳氮成键反应研究进展[J]. 有机化学, 2025, 45(5): 1460-1477. |
| [7] | 周思成, 刘运奎. P/N-杂配铜(I)光催化剂介导的可见光催化反应进展[J]. 有机化学, 2025, 45(5): 1644-1668. |
| [8] | 陈雨佳, 刘志林, 陈凯, 向皞月, 阳华. 无金属、光催化氧化苄基C—H键以获得羰基官能团[J]. 有机化学, 2025, 45(5): 1755-1762. |
| [9] | 蒋晨阳, 尹艳丽, 江智勇. 光酶催化不对称自由基加成反应研究进展[J]. 有机化学, 2025, 45(5): 1614-1633. |
| [10] | 吴利华, 杨建静, 闫克鲁, 许丽荣, 文江伟. 单原子光催化有机合成研究进展[J]. 有机化学, 2025, 45(5): 1591-1613. |
| [11] | 林风, 张艳, 吴明, 刘会艳, 郝文娟, 姜波. 利用可见光引发1,6-烯炔的增环酰化双官能化制备1-茚酮衍生物[J]. 有机化学, 2025, 45(5): 1729-1738. |
| [12] | 洪洋, 邓红平. 可见光催化的酸性C(sp3)—H键官能团化反应研究进展[J]. 有机化学, 2025, 45(5): 1569-1590. |
| [13] | 牛丽菁, 吴成娟, 梁文静, 耿琰, 董育斌. 光催化串联反应构建共价有机框架[J]. 有机化学, 2025, 45(5): 1707-1715. |
| [14] | 谭芳芳, 史孟欣, 张文敏, 李洋. 光催化生物质相关转化[J]. 有机化学, 2025, 45(5): 1523-1547. |
| [15] | 谭燕, 应佳乐, 於兵, 陆展. 可见光促进烯基硅化合物有氧氧化-叠氮化反应[J]. 有机化学, 2025, 45(5): 1684-1690. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||