Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (10): 3526-3543.DOI: 10.6023/cjoc202306007 Previous Articles Next Articles
Special Issue: 二氧化碳虚拟合辑; 有机硅化学专辑-2023
收稿日期:2023-06-11
修回日期:2023-08-07
发布日期:2023-08-30
基金资助:
Peifeng Sua, Jinyu Nia, Zhuofeng Kea,b( )
)
Received:2023-06-11
Revised:2023-08-07
Published:2023-08-30
Contact:
*E-mail: Supported by:Share
Peifeng Su, Jinyu Ni, Zhuofeng Ke. Recent Advances in Homogeneous Catalytic Systems for CO2 Hydrosilylation and Related Transformations[J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3526-3543.



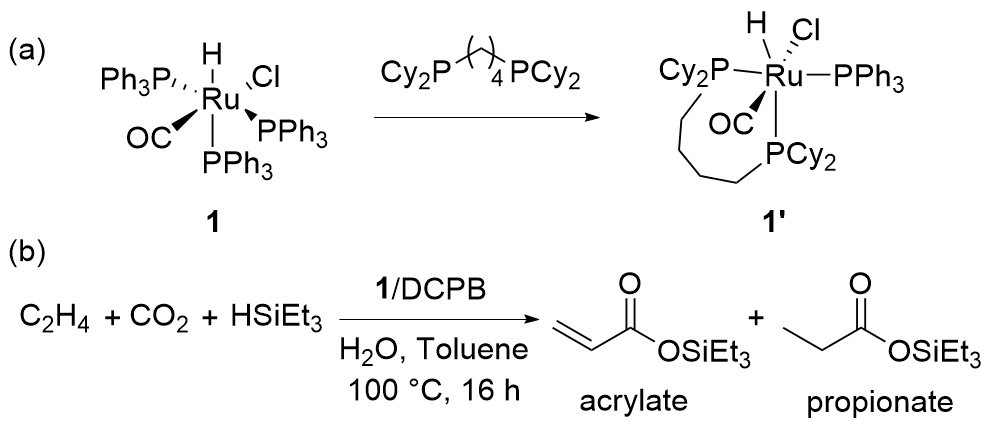

| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactant | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 1/DCPB[ | HSiEt3 | 1 | C2H4 | Toluene/H2O | 100 | 16 | Acrylate+propionate |
| 2 | 2[ | HSiMe2Ph | 0.3 | Amine | C6D6 | 50 | 14 | Silylcarbamates |
| 3 | 3/B(C6F5)3[ | HMTS, HSiMe2Ph, HSiMePh2, or HSiEt3 | 0.1 | — | C6D6 | 50 | 8~40 | Bis(silyl)acetals |
| 4 | 4[ | HSiMe(OSiMe3)2, HSiMe2Ph, or HSiMePh2 | 0.27 | — | C6D6 | 50 | 3~24 | Silylcarbonates+silylfor- mates+methoxysilanes |
| 5 | 5[ | HSiMe2Ph, HSiMePh2, or HSiEt3 | 0.3 | — | CD3CN | 75 | 2~10 | Silylformates |
| 6 | 5[ | HSiMe2Ph | 0.3 | Amine | CD3CN | 75 | 15 | Silylcarbamates |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactant | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 1/DCPB[ | HSiEt3 | 1 | C2H4 | Toluene/H2O | 100 | 16 | Acrylate+propionate |
| 2 | 2[ | HSiMe2Ph | 0.3 | Amine | C6D6 | 50 | 14 | Silylcarbamates |
| 3 | 3/B(C6F5)3[ | HMTS, HSiMe2Ph, HSiMePh2, or HSiEt3 | 0.1 | — | C6D6 | 50 | 8~40 | Bis(silyl)acetals |
| 4 | 4[ | HSiMe(OSiMe3)2, HSiMe2Ph, or HSiMePh2 | 0.27 | — | C6D6 | 50 | 3~24 | Silylcarbonates+silylfor- mates+methoxysilanes |
| 5 | 5[ | HSiMe2Ph, HSiMePh2, or HSiEt3 | 0.3 | — | CD3CN | 75 | 2~10 | Silylformates |
| 6 | 5[ | HSiMe2Ph | 0.3 | Amine | CD3CN | 75 | 15 | Silylcarbamates |


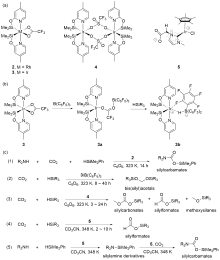
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 6[ | H3SiPh | 0.7 | — | THF | 100 | 72 | Methoxysilanes |
| 2 | Mn(CO)5Br[ | HSiEt3 | 0.4 | — | THF or THF/toluene | 50 | 1 | Silylformates+ bis(silyl)acetals |
| 3a | 7[ | H3SiPh | 0.1 | Secondary amides or lactams | MeCN | r.t. | 12 | Acyl formamides |
| 4 | 8[ | H3SiPh | 1.5 | Amines | MeCN | r.t. | 24 | Formamidesb |
| 5 | 8[ | H3SiPh | 0.1 | Amines | MeCN | r.t. | 24 | Formamides |
| 6 | 8[ | H3SiPh | 0.1 | Amines | MeCN | 100 | 15 | Tertiary amine |
| 7 | 9[ | H3SiPh | 0.1 | — | DMSO-d6 | 25 | 1~24 | Silylformates+methoxysilanes |
| 8 | 9[ | H3SiPh or H2SiPh2 | 0.1 | — | DMSO-d6 | 80 | 1~24 | Methoxysilanes |
| 9 | 10[ | H2SiMePh | 0.1 | — | C6D6 | 70 | 8 | Silanol |
| 10 | Cu(OAc)2/ mPEG-PNP[ | HSi(OMe)3 | 0.1 | Amines | Toluene | 30 | 2 | Formamides |
| 11 | Cu(OAc)2[ | H3SiPh | 0.1 | 2-(Methylamino)pyridine | Neat | 20 | 18 | Formamides |
| 12c | 12[ | H2SiPh2 | 0.1 | — | Neat | 40 | 4 | Silylformates |
| 13c | 12[ | H3SiPh | 0.1 | — | Neat | 80 | 8 | Bis(silyl)acetals |
| 14c | 12[ | H3SiPh | 0.1 | — | DMSO-d6 | 80 | 4 | Methoxysilanes |
| 15 | 13[ | H3SiPh | 0.1 | Anilines | MeCN | r.t. | 12 | Aryl formamides |
| 16 | 14/P(n-Bu)3[ | H3SiPh | 0.1 | Tryptamine derivative | THF/MeCN (V∶V=1∶1) | 60 | 36 | Tertiary amines |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 6[ | H3SiPh | 0.7 | — | THF | 100 | 72 | Methoxysilanes |
| 2 | Mn(CO)5Br[ | HSiEt3 | 0.4 | — | THF or THF/toluene | 50 | 1 | Silylformates+ bis(silyl)acetals |
| 3a | 7[ | H3SiPh | 0.1 | Secondary amides or lactams | MeCN | r.t. | 12 | Acyl formamides |
| 4 | 8[ | H3SiPh | 1.5 | Amines | MeCN | r.t. | 24 | Formamidesb |
| 5 | 8[ | H3SiPh | 0.1 | Amines | MeCN | r.t. | 24 | Formamides |
| 6 | 8[ | H3SiPh | 0.1 | Amines | MeCN | 100 | 15 | Tertiary amine |
| 7 | 9[ | H3SiPh | 0.1 | — | DMSO-d6 | 25 | 1~24 | Silylformates+methoxysilanes |
| 8 | 9[ | H3SiPh or H2SiPh2 | 0.1 | — | DMSO-d6 | 80 | 1~24 | Methoxysilanes |
| 9 | 10[ | H2SiMePh | 0.1 | — | C6D6 | 70 | 8 | Silanol |
| 10 | Cu(OAc)2/ mPEG-PNP[ | HSi(OMe)3 | 0.1 | Amines | Toluene | 30 | 2 | Formamides |
| 11 | Cu(OAc)2[ | H3SiPh | 0.1 | 2-(Methylamino)pyridine | Neat | 20 | 18 | Formamides |
| 12c | 12[ | H2SiPh2 | 0.1 | — | Neat | 40 | 4 | Silylformates |
| 13c | 12[ | H3SiPh | 0.1 | — | Neat | 80 | 8 | Bis(silyl)acetals |
| 14c | 12[ | H3SiPh | 0.1 | — | DMSO-d6 | 80 | 4 | Methoxysilanes |
| 15 | 13[ | H3SiPh | 0.1 | Anilines | MeCN | r.t. | 12 | Aryl formamides |
| 16 | 14/P(n-Bu)3[ | H3SiPh | 0.1 | Tryptamine derivative | THF/MeCN (V∶V=1∶1) | 60 | 36 | Tertiary amines |
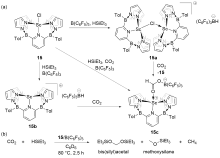
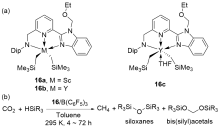
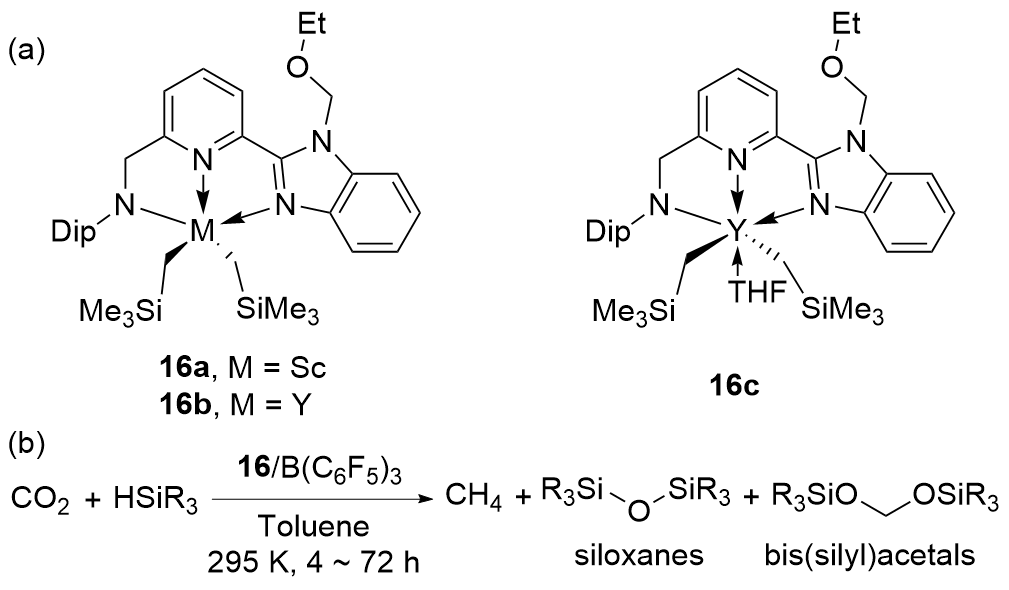
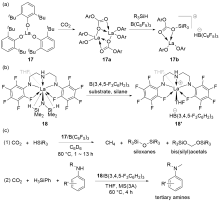

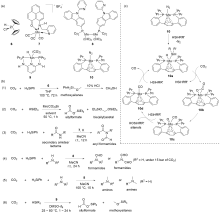
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 15/B(C6F5)3[ | HSiEt3 | 0.1 | — | C6D6 | 80 | 2.5 | Bis(silyl)acetals+ methoxysilanes+CH4 |
| 2 | 16/B(C6F5)3[ | HSiMe2Ph, H3SiPh, or HSiEt2Me | 0.1 | — | Toluene | 22 | 4~72 | Bis(silyl)acetals+silo- xanes+CH4 |
| 3 | 17/B(C6F5)3[ | HSiMePh2, H3SiPh, or HSiEt3 | 0.5 | — | C6D6 | 80 | 1~13 | Bis(silyl)acetals+silo- xanes+CH4 |
| 4a | 18/B(3,4,5-F3C6H2)3[ | H3SiPh | 0.1 | Anilines | THF | 60 | 4 | tertiary amines |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 15/B(C6F5)3[ | HSiEt3 | 0.1 | — | C6D6 | 80 | 2.5 | Bis(silyl)acetals+ methoxysilanes+CH4 |
| 2 | 16/B(C6F5)3[ | HSiMe2Ph, H3SiPh, or HSiEt2Me | 0.1 | — | Toluene | 22 | 4~72 | Bis(silyl)acetals+silo- xanes+CH4 |
| 3 | 17/B(C6F5)3[ | HSiMePh2, H3SiPh, or HSiEt3 | 0.5 | — | C6D6 | 80 | 1~13 | Bis(silyl)acetals+silo- xanes+CH4 |
| 4a | 18/B(3,4,5-F3C6H2)3[ | H3SiPh | 0.1 | Anilines | THF | 60 | 4 | tertiary amines |
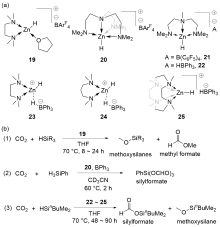



| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | Zn(OAc)2/phen[ | H2SiPh2 | 1 | — | CD3CN | r.t.~80 | 24 | Silylformates+bis(silyl)ace- tals+methoxysilanes+CH4 |
| 2 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Amines | MeCN | 25 | 4~24 | Formamidesa |
| 3 | Zn(OAc)2/phen[ | H2SiPh2 | 0.5 | Amines | MeCN | 120 | 24 | Tertiary amines |
| 4 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Amides | MeCN | 25~100 | 24 | Acyl formamides |
| 5 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Carbamates | MeCN | 25~100 | 24 | Formate formamides |
| 6 | ZnEt2[ | HSi(OEt)3 | 1 | — | Neat | 90 | 7 | Siloxanes+methoxysilanes |
| 7 | ZnEt2[ | HSi(OEt)3 | 1.5 | Secondary amines | Neat | 100 | 24 | Formamides |
| 8 | ZnEt2[ | HSi(OEt)3 | 1.5 | Primary amines | Neat | 100 | 24 | Urea derivatives |
| 9 | 19[ | HSiEtMe2 or HSinBuMe2 | 0.1 | — | THF | 70 | 8~24 | Methoxysilanes+methyl formate |
| 10b | 20[ | H3SiPh | 0.15 | — | CD3CN | 60 | 2 | silylformates |
| 11 | 22[ | HSinBuMe2 | 0.1 | — | THF | 70 | 90 | Methoxysilanes+silylformates |
| 12 | 23[ | HSinBuMe2 | 0.1 | — | THF | 70 | 90 | Methoxysilanes+silylformates |
| 13 | 24[ | HSinBuMe2 | 0.1 | — | THF | 70 | 48 | Methoxysilanes+silylformates |
| 14 | 24[ | HSinBuMe2 | 0.1 | — | THF | 70 | 72 | Methoxysilanes |
| 15 | 25[ | HSinBuMe2 | 0.1 | — | THF | 70 | 48 | Silylformates |
| 16 | 26[ | HSi[N(CH2CH2O)3] or HSi(OEt)3 | 0.1 | — | C6D6 | 100 | 24~264 | Methoxysilanes+silylformates |
| 17 | 27[ | HSi(OMe)3 or HSi(OEt)3 | 0.1 | — | C6D6 | r.t. | 72 | Silylformates |
| 18 | 28[ | HSi(OEt)3 | 0.1 | — | DMF | 60~80 | 24 | Silylformates |
| 19 | 29[ | H3SiPh | 0.1 | Anilines or indoles | Neat | 60~100 | 24~96 | Anilines or indoles derivatives |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | Zn(OAc)2/phen[ | H2SiPh2 | 1 | — | CD3CN | r.t.~80 | 24 | Silylformates+bis(silyl)ace- tals+methoxysilanes+CH4 |
| 2 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Amines | MeCN | 25 | 4~24 | Formamidesa |
| 3 | Zn(OAc)2/phen[ | H2SiPh2 | 0.5 | Amines | MeCN | 120 | 24 | Tertiary amines |
| 4 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Amides | MeCN | 25~100 | 24 | Acyl formamides |
| 5 | Zn(OAc)2/phen[ | H3SiPh | 0.5 | Carbamates | MeCN | 25~100 | 24 | Formate formamides |
| 6 | ZnEt2[ | HSi(OEt)3 | 1 | — | Neat | 90 | 7 | Siloxanes+methoxysilanes |
| 7 | ZnEt2[ | HSi(OEt)3 | 1.5 | Secondary amines | Neat | 100 | 24 | Formamides |
| 8 | ZnEt2[ | HSi(OEt)3 | 1.5 | Primary amines | Neat | 100 | 24 | Urea derivatives |
| 9 | 19[ | HSiEtMe2 or HSinBuMe2 | 0.1 | — | THF | 70 | 8~24 | Methoxysilanes+methyl formate |
| 10b | 20[ | H3SiPh | 0.15 | — | CD3CN | 60 | 2 | silylformates |
| 11 | 22[ | HSinBuMe2 | 0.1 | — | THF | 70 | 90 | Methoxysilanes+silylformates |
| 12 | 23[ | HSinBuMe2 | 0.1 | — | THF | 70 | 90 | Methoxysilanes+silylformates |
| 13 | 24[ | HSinBuMe2 | 0.1 | — | THF | 70 | 48 | Methoxysilanes+silylformates |
| 14 | 24[ | HSinBuMe2 | 0.1 | — | THF | 70 | 72 | Methoxysilanes |
| 15 | 25[ | HSinBuMe2 | 0.1 | — | THF | 70 | 48 | Silylformates |
| 16 | 26[ | HSi[N(CH2CH2O)3] or HSi(OEt)3 | 0.1 | — | C6D6 | 100 | 24~264 | Methoxysilanes+silylformates |
| 17 | 27[ | HSi(OMe)3 or HSi(OEt)3 | 0.1 | — | C6D6 | r.t. | 72 | Silylformates |
| 18 | 28[ | HSi(OEt)3 | 0.1 | — | DMF | 60~80 | 24 | Silylformates |
| 19 | 29[ | H3SiPh | 0.1 | Anilines or indoles | Neat | 60~100 | 24~96 | Anilines or indoles derivatives |

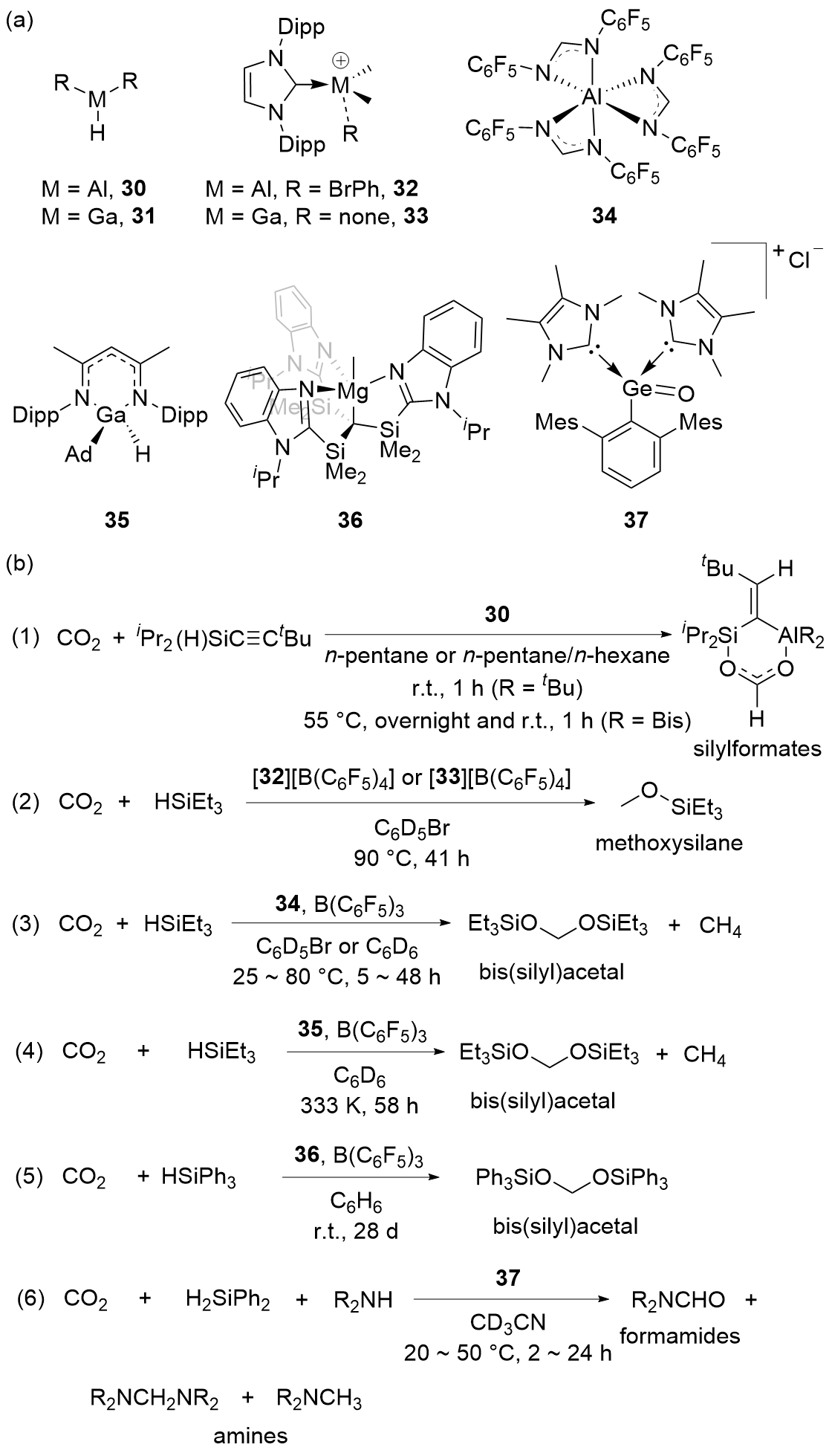
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 30[ | iPr2Si(H)C≡CtBu | 0.10 | — | n-Pentane or n- pentane/n-hexane | r.t.~55 | 1~12 | Silylformates |
| 2 | [32][B(C6F5)4][ | HSiEt3 | 0.15 | — | C6D5Br | 90 | 41 | Methoxysilanes |
| 3 | [33][B(C6F5)4][ | HSiEt3 | 0.15 | — | C6D5Br | 90 | 41 | Methoxysilanes |
| 4a | 34[ | HSiEt3 | 0.60 | — | C6D5Br or C6D6 | 25~80 | 5~48 | Bis(silyl)acetals+CH4 |
| 5a | 35[ | HSiEt3 | 0.20 | — | C6D6 | 60 | 58 | Bis(silyl)acetals+CH4 |
| 6a | 36[ | HSiPh3 | 0.10 | — | C6H6 | r.t. | 672 | Bis(silyl)acetals |
| 7 | 37[ | H2SiPh2 | 0.10 | Amines | CD3CN | 20~50 | 2~24 | Formamides+diami- nes+methyl amines |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 30[ | iPr2Si(H)C≡CtBu | 0.10 | — | n-Pentane or n- pentane/n-hexane | r.t.~55 | 1~12 | Silylformates |
| 2 | [32][B(C6F5)4][ | HSiEt3 | 0.15 | — | C6D5Br | 90 | 41 | Methoxysilanes |
| 3 | [33][B(C6F5)4][ | HSiEt3 | 0.15 | — | C6D5Br | 90 | 41 | Methoxysilanes |
| 4a | 34[ | HSiEt3 | 0.60 | — | C6D5Br or C6D6 | 25~80 | 5~48 | Bis(silyl)acetals+CH4 |
| 5a | 35[ | HSiEt3 | 0.20 | — | C6D6 | 60 | 58 | Bis(silyl)acetals+CH4 |
| 6a | 36[ | HSiPh3 | 0.10 | — | C6H6 | r.t. | 672 | Bis(silyl)acetals |
| 7 | 37[ | H2SiPh2 | 0.10 | Amines | CD3CN | 20~50 | 2~24 | Formamides+diami- nes+methyl amines |

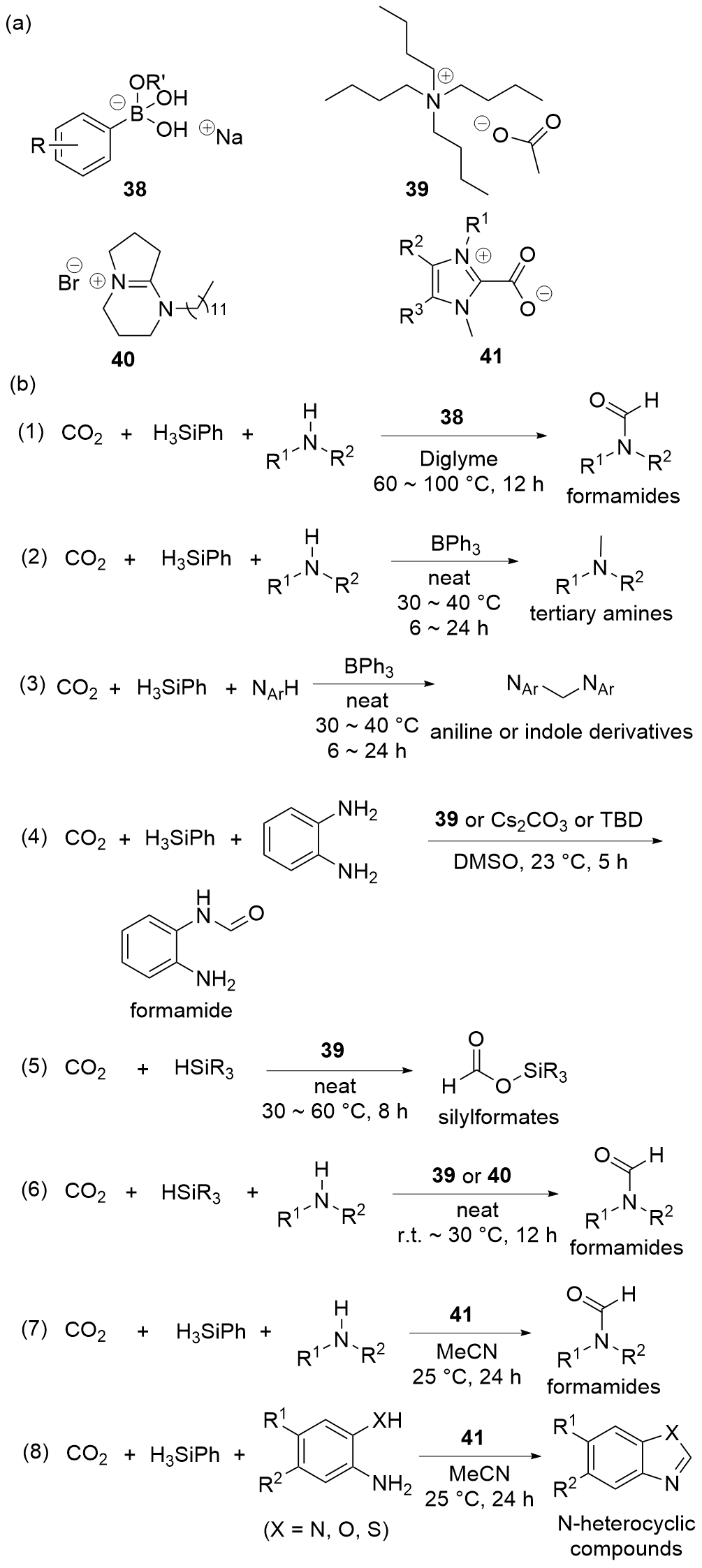
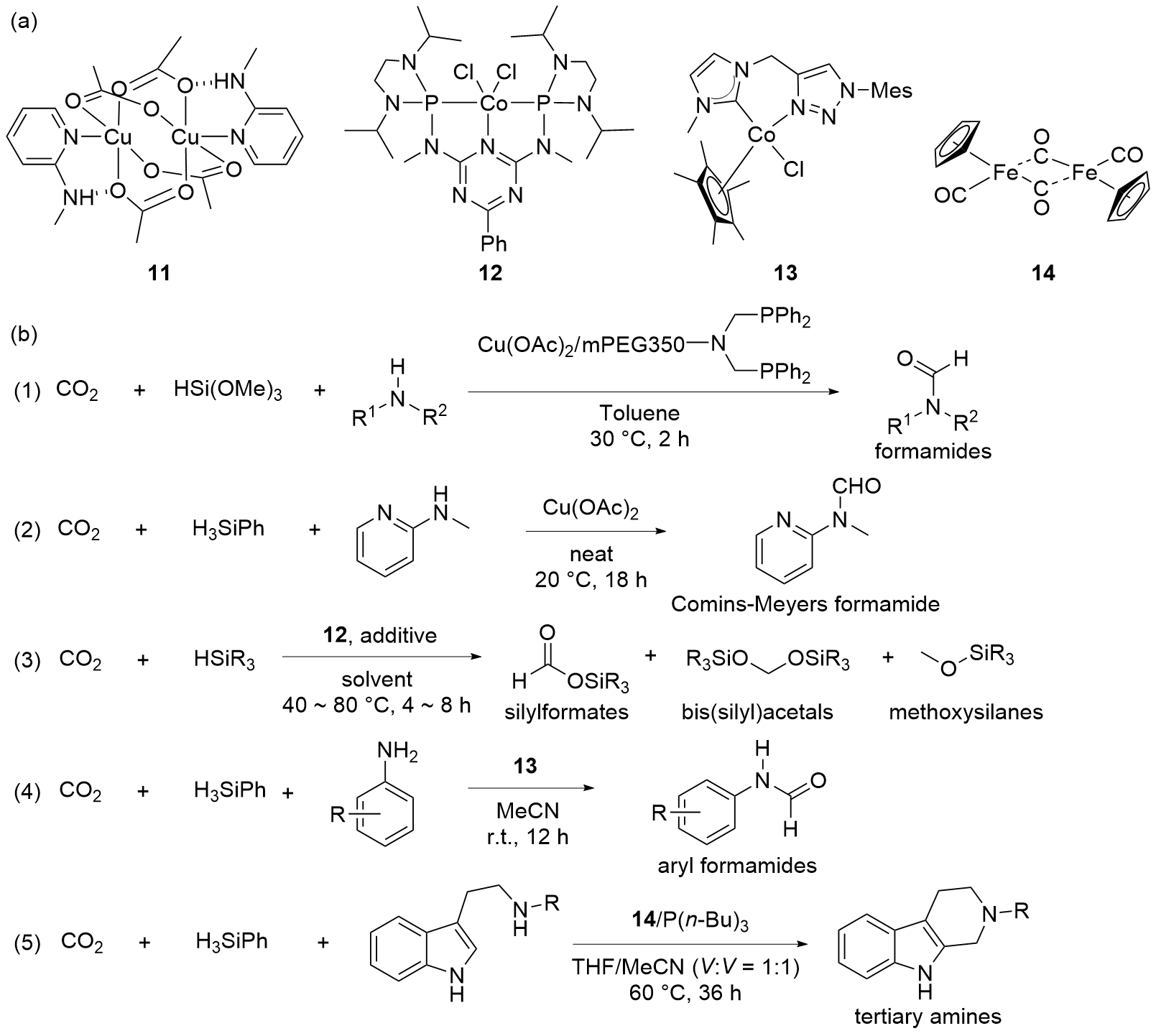
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 38[ | H3SiPh | 0.25 | Amines | Diglyme | 60~100 | 12 | Formamides |
| 2 | BPh3[ | H3SiPh | 0.1 | Amines | Neat | 30~40 | 6~24 | Tertiary amines |
| 3 | BPh3[ | H3SiPh | 0.1 | Anilines or indoles | Neat | 30~40 | 6~24 | Anilines or indoles derivatives |
| 4 | 39[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 5 | Cs2CO3[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 6 | TBD[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 7 | 39[ | HSiMe2Ph, HSiMePh2, HSiPh3, or H3SiPh | 0.1 | — | Neat | 30~60 | 8 | Silylformates |
| 8 | 39[ | H3SiPh or PMHS | 0.1 | Amines | Neat | 30 | 12 | Formamides |
| 9 | 40[ | H3SiPh | 0.5 | Amines | Neat | r.t. | 12 | Formamides |
| 10 | 41[ | H3SiPh | 0.1 | Amines | MeCN | 25 | 24 | Formamides |
| 11 | 41[ | H3SiPh | 0.1 | o-Phenylenediamines | MeCN | 25 | 24 | Benzimidazoles |
| 12 | 41[ | H3SiPh | 0.1 | o-Hydroxyaniline | MeCN | 25 | 24 | Benzoxazole |
| 13 | 41[ | H3SiPh | 0.1 | o-Mercaptonoaniline | MeCN | 25 | 24 | Benzothiazole |
| Entry | Catalyst | Silanes | p(CO2)/MPa | Other reactants | Solvent | T/℃ | t/h | Products |
|---|---|---|---|---|---|---|---|---|
| 1 | 38[ | H3SiPh | 0.25 | Amines | Diglyme | 60~100 | 12 | Formamides |
| 2 | BPh3[ | H3SiPh | 0.1 | Amines | Neat | 30~40 | 6~24 | Tertiary amines |
| 3 | BPh3[ | H3SiPh | 0.1 | Anilines or indoles | Neat | 30~40 | 6~24 | Anilines or indoles derivatives |
| 4 | 39[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 5 | Cs2CO3[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 6 | TBD[ | H3SiPh | 0.1 | Amines | DMSO | 23 | 5 | Formamides |
| 7 | 39[ | HSiMe2Ph, HSiMePh2, HSiPh3, or H3SiPh | 0.1 | — | Neat | 30~60 | 8 | Silylformates |
| 8 | 39[ | H3SiPh or PMHS | 0.1 | Amines | Neat | 30 | 12 | Formamides |
| 9 | 40[ | H3SiPh | 0.5 | Amines | Neat | r.t. | 12 | Formamides |
| 10 | 41[ | H3SiPh | 0.1 | Amines | MeCN | 25 | 24 | Formamides |
| 11 | 41[ | H3SiPh | 0.1 | o-Phenylenediamines | MeCN | 25 | 24 | Benzimidazoles |
| 12 | 41[ | H3SiPh | 0.1 | o-Hydroxyaniline | MeCN | 25 | 24 | Benzoxazole |
| 13 | 41[ | H3SiPh | 0.1 | o-Mercaptonoaniline | MeCN | 25 | 24 | Benzothiazole |
| [1] |
Schuur E. A.; McGuire A. D.; Schadel C.; Grosse G.; Harden J. W.; Hayes D. J.; Hugelius G.; Koven C. D.; Kuhry P.; Lawrence D. M.; Natali S. M.; Olefeldt D.; Romanovsky V. E.; Schaefer K.; Turetsky M. R.; Treat C. C.; Vonk J. E. Nature 2015, 520, 171.
doi: 10.1038/nature14338 |
| [2] |
Canadell J. G.; Schulze E. D. Nat. Commun. 2014, 5, 5282.
doi: 10.1038/ncomms6282 pmid: 25407959 |
| [3] |
Hotchkiss J. H.; Werner B. G.; Lee E. Y. C. Compr. Rev. Food Sci. Food Saf. 2006, 5, 158.
doi: 10.1111/crfs.2006.5.issue-4 |
| [4] |
Cuomo R.; Sarnelli G.; Savarese M. F.; Buyckx M. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 683.
doi: 10.1016/j.numecd.2009.03.020 pmid: 19502016 |
| [5] |
Singh P.; Wani A. A.; Karim A. A.; Langowski H.-C. Int. J. Dairy Technol. 2012, 65, 161.
doi: 10.1111/idt.2012.65.issue-2 |
| [6] |
Pearson A. Int. J. Refrig. 2005, 28, 1140.
doi: 10.1016/j.ijrefrig.2005.09.005 |
| [7] |
Ramsey E.; Sun Q.; Zhang Z.; Zhang C.; Gou W. J. Environ. Sci. 2009, 21, 720.
doi: 10.1016/S1001-0742(08)62330-X |
| [8] |
Zhou Y.; Zhu R.; Wei G. Powder Technol. 2021, 389, 21.
doi: 10.1016/j.powtec.2021.05.003 |
| [9] |
You X.; He S.; Zhang M.; Zeng J.; Li L.; Wang Q.; Wang Q.; Li Y. Steel Res. Int. 2019, 91, 1900450.
doi: 10.1002/srin.v91.2 |
| [10] |
Maeda C.; Miyazaki Y.; Ema T. Catal. Sci. Technol. 2014, 4, 1482.
|
| [11] |
Ye R.-P.; Ding J.; Gong W.; Argyle M. D.; Zhong Q.; Wang Y.; Russell C. K.; Xu Z.; Russell A. G.; Li Q.; Fan M.; Yao Y.-G. Nat. Commun. 2019, 10, 5698.
doi: 10.1038/s41467-019-13638-9 |
| [12] |
Artz J.; Müller T. E.; Thenert K.; Kleinekorte J.; Meys R.; Sternberg A.; Bardow A.; Leitner W. Chem. Rev. 2017, 118, 434.
doi: 10.1021/acs.chemrev.7b00435 |
| [13] |
Chauvier C.; Cantat T. ACS Catal. 2017, 7, 2107.
doi: 10.1021/acscatal.6b03581 |
| [14] |
Kostera S.; Peruzzini M.; Gonsalvi L. Catalysts 2021, 11, 58.
doi: 10.3390/catal11010058 |
| [15] |
Fernández-Alvarez F. J.; Oro L. A. ChemCatChem 2018, 10, 4783.
|
| [16] |
Chen J.; McGraw M.; Chen E. Y. ChemSusChem 2019, 12, 4543.
doi: 10.1002/cssc.v12.20 |
| [17] |
Wang X.; Xia C.; Wu L. Green Chem. 2018, 20, 5415.
doi: 10.1039/C8GC03022G |
| [18] |
Zhang Y.; Zhang T.; Das S. Green Chem. 2020, 22, 1800.
doi: 10.1039/C9GC04342J |
| [19] |
Motokura K.; Pramudita R. A. Chem. Rec. 2019, 19, 1199.
doi: 10.1002/tcr.201800076 |
| [20] |
Fernández-Alvarez F. J.; Aitani A. M.; Oro L. A. Catal. Sci. Technol. 2014, 4, 611.
doi: 10.1039/C3CY00948C |
| [21] |
Iglesias M.; Fernández-Alvarez F. J.; Oro L. A. Coord. Chem. Rev. 2019, 386, 240.
doi: 10.1016/j.ccr.2019.02.003 |
| [22] |
Zhao S.; Liang H.-Q.; Hu X.-M.; Li S.; Daasbjerg K. Angew. Chem., Int. Ed. 2022, 61, e202204008.
|
| [23] |
Liu M.; Qin T.; Zhang Q.; Fang C.; Fu Y.; Lin B.-L. Sci. China: Chem. 2015, 58, 1524.
|
| [24] |
Li B.; Sortais J.-B.; Darcel C. RSC Adv. 2016, 6, 57603.
doi: 10.1039/C6RA10494K |
| [25] |
Liu X.; Li J.; Li N.; Li B.; Bu X.-H. Chin. J. Chem. 2021, 39, 440.
doi: 10.1002/cjoc.v39.2 |
| [26] |
Jiang Y.; Li G.; Chen Q.; Xu Z.; Lin S.; Guo G. Acta Chim. Sinica 2022, 80, 703 (in Chinese).
doi: 10.6023/A22010012 |
|
(蒋银龙, 李国超, 陈青松, 徐忠宁, 林姗姗, 郭国聪, 化学学报, 2022, 80, 703.)
doi: 10.6023/A22010012 |
|
| [27] |
Wang X.; Yang X.; Chen C.; Li H.; Huang Y.; Cao R. Acta Chim. Sinica 2022, 80, 22 (in Chinese).
doi: 10.6023/A21100455 |
|
(王旭生, 杨胥, 陈春辉, 李红芳, 黄远标, 曹荣, 化学学报, 2022, 80, 22.)
doi: 10.6023/A21100455 |
|
| [28] |
Addis D.; Das S.; Junge K.; Beller M. Angew. Chem., Int. Ed. 2011, 50, 6004.
doi: 10.1002/anie.v50.27 |
| [29] |
Wang H.; Dong Y.; Zheng C.; Sandoval C. A.; Wang X.; Makha M.; Li Y. Chem 2018, 4, 2883.
doi: 10.1016/j.chempr.2018.09.009 |
| [30] |
Yan F.; Bai J.-F.; Dong Y.; Liu S.; Li C.; Du C.-X.; Li Y. JACS Au 2022, 2, 2522.
doi: 10.1021/jacsau.2c00392 |
| [31] |
Dong Y.; Yang P.; Zhao S.; Li Y. Nat. Commun. 2020, 11, 4096.
doi: 10.1038/s41467-020-17939-2 |
| [32] |
Kunihiro K.; Heyte S.; Paul S.; Roisnel T.; Carpentier J. F.; Kirillov E. Chem. Eur. J. 2021, 27, 3997.
doi: 10.1002/chem.v27.12 |
| [33] |
Guzmán J.; Torguet A.; García-Orduña P.; Lahoz F. J.; Oro L. A.; Fernández-Alvarez F. J. J. Organomet. Chem. 2019, 897, 50.
doi: 10.1016/j.jorganchem.2019.06.010 |
| [34] |
Guzmán J.; Urriolabeitia A.; Padilla M.; García-Orduña P.; Polo V.; Fernández-Alvarez F. J. Inorg. Chem. 2022, 61, 20216.
doi: 10.1021/acs.inorgchem.2c03330 |
| [35] |
Guzmán J.; García-Orduña P.; Lahoz F. J.; Fernández-Alvarez F. J. RSC Adv. 2020, 10, 9582.
doi: 10.1039/D0RA00204F |
| [36] |
Ojeda-Amador A. I.; Munarriz J.; Alamán-Valtierra P.; Polo V.; Puerta-Oteo R.; Jiménez M. V.; Fernández-Alvarez F. J.; Pérez-Torrente J. J. ChemCatChem 2019, 11, 5524.
doi: 10.1002/cctc.201901687 |
| [37] |
Roa D. A.; Garcia J. J. New J. Chem. 2023, 47, 4504.
doi: 10.1039/D2NJ06204F |
| [38] |
González T.; García J. J. Polyhedron 2021, 203, 115242.
doi: 10.1016/j.poly.2021.115242 |
| [39] |
Chakraborty S.; Nath R.; Kumar Ray A.; Paul A.; Mandal S. K. Chem. Eur. J. 2022, 28, e202202710.
|
| [40] |
Huang Z.; Jiang X.; Zhou S.; Yang P.; Du C.-X.; Li Y. ChemSusChem 2019, 12, 3054.
doi: 10.1002/cssc.v12.13 |
| [41] |
Bertini F.; Glatz M.; Stöger B.; Peruzzini M.; Veiros L. F.; Kirchner K.; Gonsalvi L. ACS Catal. 2019, 9, 632.
doi: 10.1021/acscatal.8b04106 |
| [42] |
Buss J. A.; Shida N.; He T.; Agapie T. ACS Catal. 2021, 11, 13294.
doi: 10.1021/acscatal.1c02922 |
| [43] |
Song Z.; Liu J.; Xing S.; Shao X.; Li J.; Peng J.; Bai Y. Org. Biomol. Chem. 2023, 21, 832.
doi: 10.1039/D2OB01986H |
| [44] |
Yang F.; Saiki Y.; Nakaoka K.; Ema T. Adv. Synth. Catal. 2023, 365, 877.
doi: 10.1002/adsc.v365.6 |
| [45] |
Cramer H. H.; Chatterjee B.; Weyhermuller T.; Werlé C.; Leitner W. Angew. Chem., Int. Ed. 2020, 59, 15674.
doi: 10.1002/anie.v59.36 |
| [46] |
Cramer H. H.; Ye S.; Neese F.; Werlé C.; Leitner W. JACS Au 2021, 1, 2058.
doi: 10.1021/jacsau.1c00350 pmid: 34849511 |
| [47] |
Siddique M.; Boity B.; Rit A. Organometallics 2023, 42, 1395.
doi: 10.1021/acs.organomet.2c00670 |
| [48] |
Li W.-D.; Chen J.; Zhu D.-Y.; Xia J.-B. Chin. J. Chem. 2021, 39, 614.
doi: 10.1002/cjoc.v39.3 |
| [49] |
Beh D. W.; Piers W. E.; Gelfand B. S.; Lin J.-B. Dalton Trans. 2020, 49, 95.
doi: 10.1039/C9DT04323C |
| [50] |
Gurina G. A.; Kissel A. A.; Lyubov D. M.; Luconi L.; Rossin A.; Tuci G.; Cherkasov A. V.; Lyssenko K. A.; Shavyrin A. S.; Ob'edkov A. M.; Giambastiani G.; Trifonov A. A. Dalton Trans. 2020, 49, 638.
doi: 10.1039/c9dt04338a pmid: 31819930 |
| [51] |
Chang K.; del Rosal I.; Zheng X.; Maron L.; Xu X. Dalton Trans. 2021, 50, 7804.
doi: 10.1039/D1DT01074C |
| [52] |
Shinohara K.; Tsurugi H.; Mashima K. ACS Catal. 2022, 12, 8220.
doi: 10.1021/acscatal.2c01658 |
| [53] |
Zhang Q.; Fukaya N.; Fujitani T.; Choi J.-C. Bull. Chem. Soc. Jpn. 2019, 92, 1945.
doi: 10.1246/bcsj.20190203 |
| [54] |
Zhang Q.; Lin X.-T.; Fukaya N.; Fujitani T.; Sato K.; Choi J.-C. Green Chem. 2020, 22, 8414.
doi: 10.1039/D0GC02890H |
| [55] |
Du C.; Chen Y. Acta Chim. Sinica 2020, 78, 938 (in Chinese).
doi: 10.6023/A20060268 |
|
(杜重阳, 陈耀峰. 化学学报, 2020, 78, 938.)
doi: 10.6023/A20060268 |
|
| [56] |
Ritter F.; Spaniol T. P.; Douair I.; Maron L.; Okuda J. Angew. Chem., Int. Ed. 2020, 59, 23335.
doi: 10.1002/anie.v59.51 |
| [57] |
Huang X.; Zhang K.; Shao Y.; Li Y.; Gu F.-L.; Qu L.-B.; Zhao C.; Ke Z. ACS Catal. 2019, 9, 5279.
doi: 10.1021/acscatal.9b00879 |
| [58] |
Chambenahalli R.; Bhargav R. M.; McCabe K. N.; Andrews A. P.; Ritter F.; Okuda J.; Maron L.; Venugopal A. Chem. Eur. J. 2021, 27, 7391.
doi: 10.1002/chem.v27.26 |
| [59] |
Ritter F.; Morris L. J.; McCabe K. N.; Spaniol T. P.; Maron L.; Okuda J. Inorg. Chem. 2021, 60, 15583.
doi: 10.1021/acs.inorgchem.1c02207 |
| [60] |
Ruccolo S.; Amemiya E.; Shlian D. G.; Parkin G. Can. J. Chem. 2021, 99, 259.
doi: 10.1139/cjc-2020-0451 |
| [61] |
Ruccolo S.; Sambade D.; Shlian D. G.; Amemiya E.; Parkin G. Dalton Trans. 2022, 51, 5868.
doi: 10.1039/D1DT04156H |
| [62] |
Sattler W.; Shlian D. G.; Sambade D.; Parkin G. Polyhedron 2020, 187, 114542.
doi: 10.1016/j.poly.2020.114542 |
| [63] |
Sattler W.; Parkin G. Catal. Sci. Technol. 2014, 4, 1578.
doi: 10.1039/c3cy01065a |
| [64] |
Ruccolo S.; Sattler W.; Rong Y.; Parkin G. J. Am. Chem. Soc. 2016, 138, 14542.
pmid: 27779860 |
| [65] |
Ruccolo S.; Rauch M.; Parkin G. Organometallics 2018, 37, 1708.
doi: 10.1021/acs.organomet.8b00158 |
| [66] |
Shlian D. G.; Amemiya E.; Parkin G. Chem. Commun. 2022, 58, 4188.
doi: 10.1039/D1CC06963B |
| [67] |
Baalbaki H. A.; Shu J.; Nyamayaro K.; Jung H.-J.; Mehrkhodavandi P. Chem. Commun. 2022, 58, 6192.
doi: 10.1039/D2CC01498J |
| [68] |
Takaishi K.; Kosugi H.; Nishimura R.; Yamada Y.; Ema T. Chem. Commun. 2021, 57, 8083.
doi: 10.1039/D1CC03675K |
| [69] |
Tolzmann M.; Schürmann L.; Hepp A.; Uhl W.; Layh M. Eur. J. Inorg. Chem. 2020, 4024.
|
| [70] |
Bolley A.; Specklin D.; Dagorne S. Polyhedron 2021, 194, 114956.
doi: 10.1016/j.poly.2020.114956 |
| [71] |
Huang W.; Roisnel T.; Dorcet V.; Orione C.; Kirillov E. Organometallics 2020, 39, 698.
doi: 10.1021/acs.organomet.9b00853 |
| [72] |
Caise A.; Hicks J.; Ángeles Fuentes M.; Goicoechea J. M.; Aldridge S. Chem. Eur. J. 2021, 27, 2138.
doi: 10.1002/chem.v27.6 |
| [73] |
Rauch M.; Strater Z.; Parkin G. J. Am. Chem. Soc. 2019, 141, 17754.
doi: 10.1021/jacs.9b08342 |
| [74] |
Sarkar D.; Weetman C.; Dutta S.; Schubert E.; Jandl C.; Koley D.; Inoue S. J. Am. Chem. Soc. 2020, 142, 15403.
doi: 10.1021/jacs.0c06287 |
| [75] |
Jiang X.; Huang Z.; Makha M.; Du C.-X.; Zhao D.; Wang F.; Li Y. Green Chem. 2020, 22, 5317.
doi: 10.1039/D0GC01741H |
| [76] |
Murata T.; Hiyoshi M.; Maekawa S.; Saiki Y.; Ratanasak M.; Hasegawa J.; Ema T. Green Chem. 2022, 24, 2385.
doi: 10.1039/D1GC04599G |
| [77] |
Hulla M.; Nussbaum S.; Bonnin A. R.; Dyson P. J. Chem. Commun. 2019, 55, 13089.
doi: 10.1039/C9CC06156H |
| [78] |
Murata T.; Hiyoshi M.; Ratanasak M.; Hasegawa J.; Ema T. Chem. Commun. 2020, 56, 5783.
doi: 10.1039/D0CC01371D |
| [79] |
Li X.-Y.; Fu H.-C.; Liu X.-F.; Yang S.-H.; Chen K.-H.; He L.-N. Catal. Today 2020, 356, 563.
doi: 10.1016/j.cattod.2020.01.030 |
| [80] |
Yu Z.; Li Z.; Zhang L.; Zhu K.; Wu H.; Li H.; Yang S. Green Chem. 2021, 23, 5759.
doi: 10.1039/D1GC01897C |
| [1] | Xu Liao, Zeyu Wang, Wufei Tang, Jinqing Lin. Progress in Porous Organic Polymer for Chemical Fixation of Carnbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2699-2710. |
| [2] | Zijie Song, Jun Liu, Ying Bai, Jiayun Li, Jiajian Peng. Progress in Catalysis Transformation of Carbon Dioxide through Hydrosilylation [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2068-2080. |
| [3] | Shuang Liu, Lianghua Zou, Xiaoming Wang. Advance of Dehydrogenation and Transfer Hydrogenation of Ammonia-Borane Catalyzed by Homogeneous Cobalt Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1713-1725. |
| [4] | Yongzhou Pan, Xiujin Meng, Yingchun Wang, Muxue He. Recent Progress in Electrochemical Fixation of CO2 to Construct Carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1416-1434. |
| [5] | Guijie Liu, Zhengqiang Fu, Fei Chen, Caixia Xu, Min Li, Ning Liu. N-Heterocyclic Carbene-Pyridine Manganese Complex/ Tetrabutylammonium Iodide Catalyzed Synthesis of Cyclic Carbonate from CO2 and Epoxide [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 629-635. |
| [6] | Wei Sun, Shoufei Zhu. Hydrosilylation Reactions of Alkene with Tertiary Silanes Catalyzed by Iron-Series Metals [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3339-3351. |
| [7] | Xiangqing Feng, Haifeng Du. B(C6F5)3-Catalyzed Silylation of Unsaturated Hydrocarbons [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3544-3557. |
| [8] | Fengjuan Chen, Luo Liu, Zilu Zhang, Wei Zeng. Recent Progress in Synthesis of Organosilanes Driven by Visible-Light [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3454-3469. |
| [9] | Jun Liu, Jiajian Peng, Ying Bai, Jiayun Li, Zijie Song, Peng Liu, Ting Ouyang, Huilin Lan. Progress in Photocatalytic Hydrosilylation [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3558-3568. |
| [10] | Yan Huang, Qian Zhang, Lewu Zhan, Jing Hou, Bindong Li. Hydrocarboxylation of Alkenes with Formate Salts via Photocatalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2568-2573. |
| [11] | Yong Xu, Yongxing Zhang, Jia Hu, Cheng Chen, Ye Yuan, Francis Verpoort. Synthesis of β-Oxopropylcarbamates Catalyzed by ZnO/Ionic Liquids under Atmospheric CO2 [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2542-2550. |
| [12] | Fei Chen, Sheng Tao, Ning Liu, Bin Dai. CNN-Type Binuclear Cu(I) Complexes Catalyzed Direct Carboxylation via the Fixation of CO2 at Room Temperature [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2471-2480. |
| [13] | Youcai Zhu, Xinxin Ding, Li Sun, Zhen Liu. Advances in the Production of Acrylic Acid and Its Derivatives by CO2/C2H4 Coupling [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 965-977. |
| [14] | Xin Li, Qiuling Song. Chiral Borane-Catalyzed Enantioselective Reactions [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3143-3151. |
| [15] | Peng Wang, Da Yang, Huan Liu. Recent Advances on Carbonylation of 1,3-Dienes [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3379-3389. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||