有机化学 ›› 2021, Vol. 41 ›› Issue (12): 4789-4797.DOI: 10.6023/cjoc202107033 上一篇 下一篇
研究论文
收稿日期:2021-07-15
修回日期:2021-08-20
发布日期:2021-09-02
通讯作者:
刘岩, 刘平
基金资助:
Yali Liu, Zhen Yang, Yang Li, Yan Liu( ), Ping Liu(
), Ping Liu( )
)
Received:2021-07-15
Revised:2021-08-20
Published:2021-09-02
Contact:
Yan Liu, Ping Liu
Supported by:文章分享
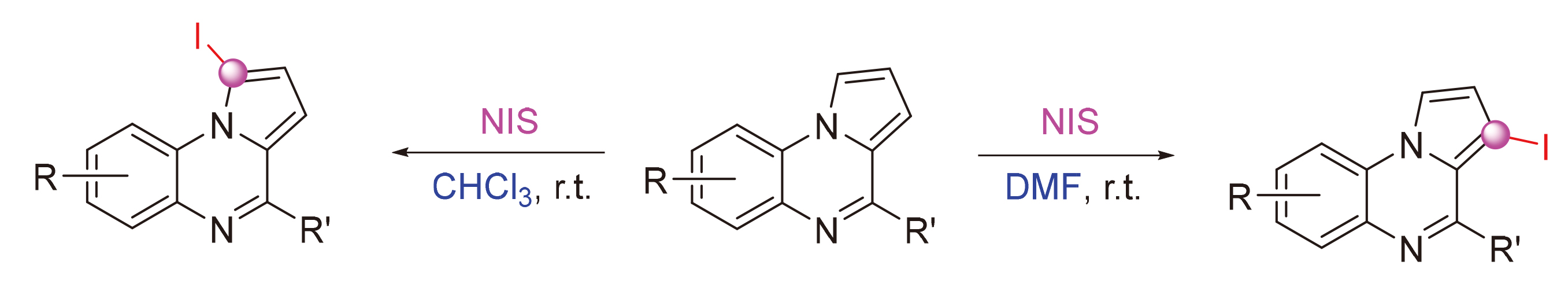
开发了一种溶剂介导吡咯并[1,2-a]喹喔啉和N-碘代丁二酰亚胺(NIS)的区域选择性C—H碘化, 分别以CHCl3和N,N-二甲基甲酰胺(DMF)为溶剂, 选择性地生成了1-碘吡咯并[1,2-a]喹喔啉和3-碘吡咯并[1,2-a]喹喔啉. 此外,吡咯并[1,2-a]喹喔啉与N-溴代丁二酰亚胺(NBS)的溴代反应得到主要产物为1,3-二溴吡咯并喹喔啉. 该方法具有反应条件简单温和、区域选择性好、底物范围广、克级合成等特点. 此外,还通过钯和碘催化的C—X (X=C, S)键形成反应研究了卤化吡咯并[1,2-a]喹喔啉产物的进一步转化.
刘亚丽, 杨振, 李阳, 刘岩, 刘平. 溶剂介导吡咯并[1,2-a]喹喔啉选择性C—H键碘代[J]. 有机化学, 2021, 41(12): 4789-4797.
Yali Liu, Zhen Yang, Yang Li, Yan Liu, Ping Liu. Solvent Mediated Selective C—H Bond Iodination of Pyrrolo[1,2-a]quinoxaline[J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4789-4797.
| Entry | [I] (equiv.) | Solvent | t/h | Yieldb/% | |
|---|---|---|---|---|---|
| 2a | 3a | ||||
| 1c | NIS (1.2) | CHCl3 | 24 | 17 | — |
| 2 | NIS (1.2) | CHCl3 | 24 | 81 | — |
| 3 | NIS (1.2) | CHCl3 | 12 | 76 | — |
| 4 | I2 (1.2) | CHCl3 | 12 | nr | — |
| 5 | TBAI (1.2) | CHCl3 | 12 | nr | — |
| 6 | NIS (1) | DMF | 6 | — | 72 |
| 7 | NIS (1) | DMSO | 6 | — | 56 |
| 8 | NIS (1) | MeCN | 6 | — | 52 |
| 9 | NIS (1) | EtOH | 6 | — | 51 |
| 10 | NIS (1) | MeOH | 6 | — | 39 |
| 11 | NIS (1) | DCM | 6 | — | trace |
| 12 | NIS (1) | Toluene | 6 | — | nr |
| 13 | I2 (1) | DMF | 6 | — | 21 |
| 14 | NIS (2) | DMF | 6 | — | 40 |
| 15 | NIS (1) | DMF | 10 | — | 73 |
| 16 | NIS (1) | DMF | 3 | — | 76 |
| Entry | [I] (equiv.) | Solvent | t/h | Yieldb/% | |
|---|---|---|---|---|---|
| 2a | 3a | ||||
| 1c | NIS (1.2) | CHCl3 | 24 | 17 | — |
| 2 | NIS (1.2) | CHCl3 | 24 | 81 | — |
| 3 | NIS (1.2) | CHCl3 | 12 | 76 | — |
| 4 | I2 (1.2) | CHCl3 | 12 | nr | — |
| 5 | TBAI (1.2) | CHCl3 | 12 | nr | — |
| 6 | NIS (1) | DMF | 6 | — | 72 |
| 7 | NIS (1) | DMSO | 6 | — | 56 |
| 8 | NIS (1) | MeCN | 6 | — | 52 |
| 9 | NIS (1) | EtOH | 6 | — | 51 |
| 10 | NIS (1) | MeOH | 6 | — | 39 |
| 11 | NIS (1) | DCM | 6 | — | trace |
| 12 | NIS (1) | Toluene | 6 | — | nr |
| 13 | I2 (1) | DMF | 6 | — | 21 |
| 14 | NIS (2) | DMF | 6 | — | 40 |
| 15 | NIS (1) | DMF | 10 | — | 73 |
| 16 | NIS (1) | DMF | 3 | — | 76 |
| [1] |
(a) Ronga, L.; Del Favero, M.; Cohen, A.; Soum, C.; Le, Pape P.; Savrimoutou, S.; Pinaud, N.; Mullie, C.; Daulouede, S.; Vincendeau, P.; Farvacques, N.; Agnamey, P.; Pagniez, F.; Hutter, S.; Azas, N.; Sonnet, P.; Guillon, J. Eur. J. Med. Chem. 2014, 81, 378.
doi: 10.1016/j.ejmech.2014.05.037 pmid: 21458112 |
|
(b) van Heerden, L.; Cloete, T. T.; Breytenbach, J. W.; de Kock, C.; Smith, P.; Breytenbach, J. C.; N’Da, D. D. Eur. J. Med. Chem. 2012, 55, 335.
doi: 10.1016/j.ejmech.2012.07.037 pmid: 21458112 |
|
|
(c) Guillon, J.; Mouray, E.; Moreau, S.; Mullie, C.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Pinaud, N.; Ravanello, F.; Le-Naour, A.; Le-Naour, Leger, J.-M.; Gosmann, G.; Jarry, C.; Deleris, G.; Sonnet, P.; Grellier, P. Eur. J. Med. Chem. 2011, 46, 2310.
doi: 10.1016/j.ejmech.2011.03.014 pmid: 21458112 |
|
|
(d) Jonet, A.; Guillon, J.; Mullie, C.; Cohen, A.; Bentzinger, G.; Schneider, J.; Taudon, N.; Hutter, S.; Azas, N.; Moreau, S.; Savrimoutou, S.; Agnamey, P.; Dassonville-Klimpt, A.; Sonnet, P. Med. Chem. 2018, 14, 293.
doi: 10.2174/1573406413666170726123938 pmid: 21458112 |
|
|
(e) Guillon, J.; Cohen, A.; Gueddouda, N. M.; Das, R. N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; Rubio, S.; Garnerin, T.; Azas, N.; Mergny, J.-L.; Mullie, C.; Sonnet, P. J. Enzyme Inhib. Med. Chem. 2017, 32, 547.
doi: 10.1080/14756366.2016.1268608 pmid: 21458112 |
|
| [2] |
Xu, H.; Fan, L. Eur. J. Med. Chem., 2011, 46, 1919.
doi: 10.1016/j.ejmech.2011.02.035 |
| [3] |
Morelli, E.; Gemma, S.; Budriesi, R.; Campiani, G.; Novellino, E.; Fattorusso, C.; Catalanotti, B.; Coccone, S. S.; Ros, S.; Borrelli, G.; Kumar, V.; Persico, M.; Fiorini, I.; Nacci, V.; Ioan, P.; Chiarini, A.; Hamon, M.; Cagnotto, A.; Mennini, T.; Fracasso, C.; Colovic, M.; Caccia, S.; Butini, S. J. Med. Chem. 2009, 52, 3548.
doi: 10.1021/jm900018b pmid: 19425598 |
| [4] |
(a) Guillon, J.; Le Borgne, M.; Rimbault, C.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Baratin, S.; Marchivie, M.; Roche, S.; Bollacke, A.; Pecci, A.; Alvarez, L.; Despla, V.; Jose, J. Eur. J. Med. Chem. 2013, 65, 205.
doi: 10.1016/j.ejmech.2013.04.051 pmid: 23711832 |
|
(b) Desplat, V.; Moreau, S.; Gay, A.; Fabre, S. B.; Thiolat, D.; Massip, S.; Macky, G.; Godde, F.; Mossalayi, D.; Jarry, C.; Guillon, J. J. Enzyme Inhib. Med. Chem. 2010, 25, 204.
doi: 10.3109/14756360903169881 pmid: 23711832 |
|
| [5] |
(a) Brindisi, M.; Brogi, S.; Maramai, S.; Grillo, A.; Borrelli, G.; Butini, S.; Novellino, E.; Allara, M.; Ligresti, A.; Campiani, G.; Di Marzo, V.; Gemma, S. RSC Adv. 2016, 6, 64651.
doi: 10.1039/C6RA12524G |
|
(b) Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S.; Sonnet, P.; Rossi, F.; Ronga, L.; Guillon, J. Eur. J. Med. Chem. 2016, 113, 214.
doi: 10.1016/j.ejmech.2016.02.047 |
|
|
(c) Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Rubio, S.; Pinaud, N.; Bigat, D.; Enriquez, E.; Marchivie, M.; Routier, S.; Sonnet, P.; Rossi, F.; Ronga, L.; Guillon, J. Chem- MedChem 2017, 12, 940.
|
|
|
(d) Wang, T.; Tang, Y.; Yang, Y.; An, Q.; Sang, Z.; Yang, T.; Liu, P.; Zhang, T.; Deng, Y.; Luo, Y.; Bioorg. Med. Chem. Lett. 2018, 28, 2084.
doi: 10.1016/j.bmcl.2018.04.043 |
|
|
(e) Gemma, S.; Colombo, L.; Forloni, G.; Savini, L.; Fracasso, C.; Caccia, S.; Salmona, M.; Brindisi, M.; Joshi, B. P.; Tripaldi, P.; Giorgi, O.; Taglialatela-Scafati, E.; Novellino, I.; Fiorini, G.; Campiani, S.; Butini, S. Org. Biomol. Chem. 2011, 9, 5137.
doi: 10.1039/c1ob05288h |
|
| [6] |
(a) Huang, A.; Ma, C. Mini-Rev. Med. Chem. 2013, 13, 607.
doi: 10.2174/1389557511313040012 pmid: 11462972 |
|
(b) Campiani, G.; Aiello, F.; Fabbrini, M.; Morelli, E.; Ramunno, A.; Armoroli, S.; Nacci, V.; Garofalo, A.; Greco, G.; Novellino, E.; Maga, G.; Spadari, S.; Bergamini, A.; Ventura, L.; Bongiovanni, B.; Capozzi, M.; Bolacchi, F.; Marini, S.; Coletta, M.; Guiso, G.; Caccia, S. J. Med. Chem. 2001, 44, 305.
pmid: 11462972 |
|
|
(c) Glennon, R. A.; Daoud, M. K.; Dukat, M.; Teitler, M.; Herrick-Davis, K.; Purohit, A.; Syed, H. Bioorg. Med. Chem. 2003, 11, 4449.
doi: 10.1016/S0968-0896(03)00488-7 pmid: 11462972 |
|
|
(d) Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P. L.; Mollicotti, P.; Sechi, L.; Zanetti, S. Eur. J. Med. Chem. 2004, 39, 195.
doi: 10.1016/j.ejmech.2003.11.008 pmid: 11462972 |
|
|
(e) Guillon, J.; Forfar, I.; Mamani-Matsuda, M.; Desplat, V.; Saliège, M.; Thiolat, D.; Massip, S.; Tabourier, A.; Léger, J.-M.; Dufaure, B.; Haumont, G.; Jarry, C.; Mossalayi, D. Bioorg. Med. Chem. 2007, 15, 194.
doi: 10.1016/j.bmc.2006.09.068 pmid: 11462972 |
|
|
(f) Moarbess, G.; Deleuze-Masquefa, C.; Bonnard, V.; Gayraud-Paniagua, S.; Vidal, J. R.; Bressolle, F.; Pinguet, P.; Bonnet, P. A. Bioorg. Med. Chem. 2008, 16, 6601.
doi: 10.1016/j.bmc.2008.05.022 pmid: 11462972 |
|
| [7] |
(a) Kalinin, A. A.; Islamova, L. N.; Fazleeva, G. M. Chem. Heterocycl. Compd. 2019, 55, 584.
doi: 10.1007/s10593-019-02501-w |
|
(b) Cong, W.; Wang, L.; Yu, F.; Li, J. Chin. J. Org. Chem. 2018, 38, 2866.
doi: 10.6023/cjoc201805046 |
|
| [8] |
(a) Qin, Y.; Zhu, L.; Luo, S. Chem. Rev. 2017, 117, 9433.
doi: 10.1021/acs.chemrev.6b00657 |
|
(b) Ping, L.; Chung, D. S.; Bouffard, J.; Lee, S. G. Chem. Soc. Rev. 2017, 46, 4299.
doi: 10.1039/C7CS00064B |
|
|
(c) Liu, C. X.; Gu, Q.; You, S. L. Trends Chem. 2020, 2, 737.
doi: 10.1016/j.trechm.2020.05.003 |
|
|
(d) Hartwig, J. F.; Larsen, M. A. ACS Cent. Sci. 2017, 46, 4299.
|
|
|
(e) Zheng, Q.; Jiao, N. Chem. Soc. Rev. 2016, 45, 4590.
doi: 10.1039/C6CS00107F |
|
|
(f) Sun, K.; Xiao, F.; Yu, B.; He, W. Chin. J. Catal. 2021, 13, 63850.
|
|
|
(g) Xie, L.; Peng, S.; Yang, L.; Peng, C.; Lin, Y.; Yu, X.; Cao, Z.; Peng, Y.; He, W. Green Chem. 2021, 23, 374.
doi: 10.1039/D0GC02844D |
|
|
(h) Chen, J.; Wu, H.; Gui, Q.; Yan, S.; Deng, J.; Lin, Y.; Cao, Z.; He, W. Chin. J. Catal. 2021, 42, 1445.
doi: 10.1016/S1872-2067(20)63750-0 |
|
|
(i) Chen, J.; Zhong, C.; Gui, Q.; Zhou, Y.; Fang, Y.; Liu, K.; Lin, Y.; Cao, Z.; He, W. Chin. Chem. Lett. 2021, 32, 475.
doi: 10.1016/j.cclet.2020.09.034 |
|
|
(j) Zhu, X.; Li, X.; Li, X. Org. Chem. Front. 2021, 8, 3128.
doi: 10.1039/D1QO00210D |
|
|
(k) Chen, Q; Yang, Y; Wang, X. Chin. J. Org. Chem. 2020, 40, 454. (in Chinese)
doi: 10.6023/cjoc201907046 |
|
|
( 陈倩雯, 杨耀成, 王霞, 有机化学, 2020, 40, 454.)
doi: 10.6023/cjoc201907046 |
|
|
(l) Hao, W.; Wang, Y.; Yang, G.; Liu, Y. Chin. J. Org. Chem. 2017, 37, 2678. (in Chinese)
|
|
|
( 郝文燕, 王昱赟, 杨国敏, 刘云云, 有机化学, 2017, 37, 2678.)
doi: 10.6023/cjoc201704049 |
|
|
(m) Gan, Z.; Li, G.; Yang, X. Sci. China. Chem. 2020, 63, 1652.
doi: 10.1007/s11426-020-9811-6 |
|
|
(n) Sun, K.; Lv, Y.; Wang, J. Org. Lett. 2015, 17, 4408.
doi: 10.1021/acs.orglett.5b01857 |
|
|
(o) Luo, J.; Xu, X.; Zhao, Y. Chin. J. Org. Chem. 2017, 37, 2873. (in Chinese)
doi: 10.6023/cjoc201705018 |
|
|
( 骆钧飞, 徐星, 赵延超, 梁洪泽, 有机化学, 2017, 37, 2873.)
doi: 10.6023/cjoc201705018 |
|
| [9] |
Yang, Z.; He, J.; Wei, Y.; Li, W.; Liu, P.; Org. Biomol. Chem. 2020, 18, 3360.
doi: 10.1039/D0OB00494D |
| [10] |
Yang, Z.; He, J.; Wei, Y.; Li, W.; Liu, P.; Zhao, J.; Wei, Y. Org. Biomol. Chem. 2020, 18, 9088.
doi: 10.1039/D0OB01818J |
| [11] |
Le, H.; Hoang, T.; Tran, T.; Nguyen, C.; Chiem, L.; Phan, N.; Nguyen, T. Tetrahedron Lett. 2021, 67, 152879.
doi: 10.1016/j.tetlet.2021.152879 |
| [12] |
Liu, Y.; Wei, Y.; Yang, Z.; Li, Y.; Liu, Y.; Liu, P. Org. Biomol. Chem. 2021, 19, 5191.
doi: 10.1039/D1OB00759A |
| [13] |
Cheeseman, G. W. H.; Roy, P. D. J. Chem. Soc. C, 1968, 2848.
|
| [14] |
Reeves, J. T.; Fandrick, D. R.; Tan, Z.; Song, J. J.; Lee, H.; Yee, N. K.; Senanayake, C. H. J. Org. Chem. 2010, 75, 992.
doi: 10.1021/jo9025644 |
| [1] | 付雅彤, 孙超凡, 张丹, 金成国, 陆居有. 巢式-碳硼烷硼氢键官能化反应研究进展[J]. 有机化学, 2024, 44(2): 438-447. |
| [2] | 张剑, 梁万洁, 杨艺, 闫法超, 刘会. 联烯胺化合物的区域选择性双官能团化[J]. 有机化学, 2024, 44(2): 335-348. |
| [3] | 范威. O2促进下五元环烯胺的C—H亚胺化[J]. 有机化学, 2023, 43(7): 2492-2498. |
| [4] | 户晓兢, 郭斐翔, 朱润青, 周柄棋, 张涛, 房立真. 对烷氧基酚的合成及其去芳构化后的合成应用[J]. 有机化学, 2023, 43(6): 2239-2244. |
| [5] | 纪健, 刘进华, 管丛, 陈绪文, 赵芸, 刘顺英. 原位生成的磺酸催化N-磺酰基-1,2,3-三氮唑与醇偶联高区域选择性合成N2-取代1,2,3-三氮唑[J]. 有机化学, 2023, 43(3): 1168-1176. |
| [6] | 沈梦涵, 李来强, 周泉, 王洁慧, 王磊. 可见光诱导下喹喔啉酮与吡咯衍生物的氧化偶联[J]. 有机化学, 2023, 43(2): 697-704. |
| [7] | 孙婧, 张萌萌, 锅小龙, 王琪, 王陆瑶. 无过渡金属条件下二芳基硒化合物的合成[J]. 有机化学, 2023, 43(12): 4251-4260. |
| [8] | 肖朵朵, 张建涛, 周鹏, 刘卫兵. 无金属条件下芳基酮与二甲亚砜的α-C(sp3)—H亚甲基化反应合成γ-酮亚砜[J]. 有机化学, 2023, 43(11): 3900-3906. |
| [9] | 彭菊, 何晓倩, 廖黎丽, 白若鹏, 蓝宇. 取代基电性效应对碳硅还原消除区域选择性调控的理论研究[J]. 有机化学, 2023, 43(10): 3608-3613. |
| [10] | 徐琳琳, 兰美君, 张慕雨, 张永琪, 冯宇豪, 荣良策, 张金鹏. 芳基乙烯β-H区域选择性三氟甲基磺酰化反应[J]. 有机化学, 2022, 42(7): 2134-2139. |
| [11] | 孙天义, 张依凡, 孟远倢, 王怡, 朱琦峰, 姜玉新, 刘石惠. 可见光-铜共催化的糖类区域选择性氧烷基化反应[J]. 有机化学, 2022, 42(5): 1414-1422. |
| [12] | 马文静, 朱礼志, 章梦珣, 李志成. ent-Kaurene全碳骨架中AB环系的不对称合成[J]. 有机化学, 2022, 42(2): 580-589. |
| [13] | 黄云帅, 靳小慧, 张凤莲, 汪义丰. 4-二甲胺基吡啶-硼自由基促进的缺电子烯烃区域选择性硼氢化反应[J]. 有机化学, 2021, 41(5): 1957-1967. |
| [14] | 韩博士, 时郑, 何慧红, 张兴华. 铜催化芳基(或烷基)卤化物选择性烯丙基化反应研究[J]. 有机化学, 2021, 41(2): 695-701. |
| [15] | 宁资慧, 彭欣华, 白瑞, 刘珊珊, 李卓, 焦林郁. 铱催化的苯甲酰胺和磷酰叠氮在离子液体中的C—H键胺化反应[J]. 有机化学, 2021, 41(11): 4484-4492. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||