有机化学 ›› 2024, Vol. 44 ›› Issue (10): 3091-3105.DOI: 10.6023/cjoc202406044 上一篇 下一篇
所属专题: 二氧化碳专题合集
综述与进展
许立锋a, 武安国a, 于芳羽a, 李红茹a,b,*( ), 何良年a,*(
), 何良年a,*( )
)
收稿日期:2024-06-28
修回日期:2024-07-24
发布日期:2024-08-30
基金资助:
Lifeng Xua, Anguo Wua, Fangyu Yua, Hongru Lia,b,*( ), Liangnian Hea,*(
), Liangnian Hea,*( )
)
Received:2024-06-28
Revised:2024-07-24
Published:2024-08-30
Contact:
*E-mail: Supported by:文章分享

环状碳酸酯是重要的化工产品, 在电池电解液、化妆品、油漆等领域具有广泛应用, 同时还是有机合成的绿色溶剂和反应原料. 环状碳酸酯是工业规模CO2转化的重要产品, 主要通过环氧化物与CO2的环加成反应进行制备. 在传统的热催化反应中, 由于环氧化物活化开环具有较高的能垒, 因而一般需要较高的反应温度. 近年来, 新材料的发展推动了环加成反应催化剂的创新, 新的催化模式不断出现, 尤其是光驱动和电驱动的环加成反应实现了温和条件下环状碳酸酯的制备. 此外, 电驱动的烯烃或邻二醇与CO2合成环状碳酸酯也取得了初步的研究成果. 总结了光/电驱动的CO2合成环状碳酸酯的反应, 旨在通过对材料设计和催化机理的介绍, 为新型反应路径和催化材料的设计提供新思路.
许立锋, 武安国, 于芳羽, 李红茹, 何良年. 可再生能源驱动的CO2基环状碳酸酯合成研究进展[J]. 有机化学, 2024, 44(10): 3091-3105.
Lifeng Xu, Anguo Wu, Fangyu Yu, Hongru Li, Liangnian He. Progress on Renewable Energy-Driven Synthesis of Cyclic Carbonates from CO2[J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3091-3105.
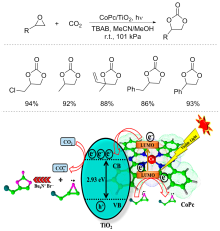



| Entry | Epoxide (n/mmol) | Catalyst (dosage) | TBAB/mol% | Solvente (V/mL) | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Propylene oxide (1) | CoPc/TiO2 (100 mg) | 10 | S1/S2 (10/5) | 24 h, 20 W LED | 92 | [ |
| 2 | Propylene oxide (3) | Ti18Bi4O29Bz26 (20 μmol) | 1.6 | 14 h, 300 W Xe lamp | >99 | [ | |
| 3b | Propylene oxide (1.4) | BiNbO4/r-GO (50 mg) | 2 | S1/S2 (10/2) | 24 h, 300 W Hg lamp | 65 | [ |
| 4c | Propylene oxide (1.4) | FeNbO4/r-GO (50 mg) | 2 | S1/S2 (4/1) | 24 h, 500 W Hg lamp | 57 | [ |
| 5 | Phenyl oxirane (44) | W18O49/g-C3N4 (30 mg) | 2.5 | 4 h, 300 W Xe lamp | 60 | [ | |
| 6 | Propylene oxide (71) | g-C3N4/Ag (10 mg) | 0.7 | 6 h, 300 W Xe lamp | [ | ||
| 7 | Phenyl oxirane (0.15) | Pd/SCNT-500 (10 mg) | 67 | 24 h, 0.15w/cm2 455nm | 97 | [ | |
| 8d | Propylene oxide (10) | Zr-Thia/g-CN (50 mg) | 2.5 | 30 h, 250w Hg lamp | 90.8 | [ | |
| 9c | Propylene oxide (1.4) | BiNbO4/NH2-MIL-125(Ti) (50 mg) | 2 | S1/S2 (5/1) | 72 h, 300 W Hg lamp | 74 | [ |
| 10c | Propylene oxide (1.4) | FeNbO4/NH2-MIL-125(Ti) (50 mg) | 2 | S1/S2 (4/1) | 72 h, 300 W Hg lamp | 52 | [ |
| 11 | Propylene oxide (4.5) | Bi-PCN-224 (30 mg) | 11 | 6 h, 300 W Xe lamp | 99 | [ | |
| 12 | Propylene oxide (0.1) | UiO-bpydc (Zn) (20 mg) | 500 | S3 (3) | 9 h, 300 W Xe lamp | 90 | [ |
| 13 | Epichlorohydrin (20) | PCN-224 (Mg) (0.5 mmol) | 1 | 6 h, LED light (3*30 W) | 99 | [ | |
| 14 | Epichlorohydrin (38) | Fe-BDC (15 mg) | 0.8 | 4 h, 300 W Xe lamp | 45 | [ | |
| 15 | Epichlorohydrin (12.75) | Fe-DBP (10 mg) | 8 | 12 h, 1000 W Xe lamp. | 97 | [ | |
| 16 | Epichlorohydrin (0.2) | TpPa-1 (5 mg) | 5 | S1 (5) | 8 h, Blue light 455nm | 82 | [ |
| 17 | Propylene oxide (0.25) | OH-P [5]-on-COF (1.5 mg) | 5 | 8 h, LED lamp | 99 | [ | |
| 18 | Epichlorohydrin (1) | 9,10-Anthraquinone (10 mol%) | 10 | S1 (10) | 12 h, 20 W LED | 93 | [ |
| Entry | Epoxide (n/mmol) | Catalyst (dosage) | TBAB/mol% | Solvente (V/mL) | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Propylene oxide (1) | CoPc/TiO2 (100 mg) | 10 | S1/S2 (10/5) | 24 h, 20 W LED | 92 | [ |
| 2 | Propylene oxide (3) | Ti18Bi4O29Bz26 (20 μmol) | 1.6 | 14 h, 300 W Xe lamp | >99 | [ | |
| 3b | Propylene oxide (1.4) | BiNbO4/r-GO (50 mg) | 2 | S1/S2 (10/2) | 24 h, 300 W Hg lamp | 65 | [ |
| 4c | Propylene oxide (1.4) | FeNbO4/r-GO (50 mg) | 2 | S1/S2 (4/1) | 24 h, 500 W Hg lamp | 57 | [ |
| 5 | Phenyl oxirane (44) | W18O49/g-C3N4 (30 mg) | 2.5 | 4 h, 300 W Xe lamp | 60 | [ | |
| 6 | Propylene oxide (71) | g-C3N4/Ag (10 mg) | 0.7 | 6 h, 300 W Xe lamp | [ | ||
| 7 | Phenyl oxirane (0.15) | Pd/SCNT-500 (10 mg) | 67 | 24 h, 0.15w/cm2 455nm | 97 | [ | |
| 8d | Propylene oxide (10) | Zr-Thia/g-CN (50 mg) | 2.5 | 30 h, 250w Hg lamp | 90.8 | [ | |
| 9c | Propylene oxide (1.4) | BiNbO4/NH2-MIL-125(Ti) (50 mg) | 2 | S1/S2 (5/1) | 72 h, 300 W Hg lamp | 74 | [ |
| 10c | Propylene oxide (1.4) | FeNbO4/NH2-MIL-125(Ti) (50 mg) | 2 | S1/S2 (4/1) | 72 h, 300 W Hg lamp | 52 | [ |
| 11 | Propylene oxide (4.5) | Bi-PCN-224 (30 mg) | 11 | 6 h, 300 W Xe lamp | 99 | [ | |
| 12 | Propylene oxide (0.1) | UiO-bpydc (Zn) (20 mg) | 500 | S3 (3) | 9 h, 300 W Xe lamp | 90 | [ |
| 13 | Epichlorohydrin (20) | PCN-224 (Mg) (0.5 mmol) | 1 | 6 h, LED light (3*30 W) | 99 | [ | |
| 14 | Epichlorohydrin (38) | Fe-BDC (15 mg) | 0.8 | 4 h, 300 W Xe lamp | 45 | [ | |
| 15 | Epichlorohydrin (12.75) | Fe-DBP (10 mg) | 8 | 12 h, 1000 W Xe lamp. | 97 | [ | |
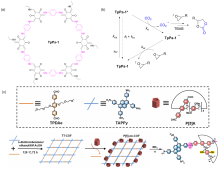
| 16 | Epichlorohydrin (0.2) | TpPa-1 (5 mg) | 5 | S1 (5) | 8 h, Blue light 455nm | 82 | [ |
| 17 | Propylene oxide (0.25) | OH-P [5]-on-COF (1.5 mg) | 5 | 8 h, LED lamp | 99 | [ | |
| 18 | Epichlorohydrin (1) | 9,10-Anthraquinone (10 mol%) | 10 | S1 (10) | 12 h, 20 W LED | 93 | [ |
| Entry | Catalystb | Zn form | Reaction condition | Light intensitya | Product | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | HPC-800 | Zn atom | 100 kPa CO2, 10 h | 300 mW•cm–2 | 4-Bromomethyl-1,3-dioxolan-2-one | 94 | [ |
| 2 | Zn SA-NC | Zn atom | 100 kPa CO2, 16 h | 300 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 99 | [ |
| 3 | ZNC-800 | Zn atom | 100 kPa CO2, 10 h | 1000 mW•cm–2 | 4-Methyl-1,3-dioxolan-2-one | 94 | [ |
| 4 | Zn-Asp-300 | ZnO | 100 kPa CO2, 4 h | 152.5 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 92 | [ |
| 5 | ZNPC-600 | ZnO | 100 kPa CO2, 6 h | 500 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 99 | [ |
| 6 | ZnS / NPC-2 | ZnS | 100 kPa CO2, 12 h | 300 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 98 | [ |
| 7 | ZnO/NC-L | ZnO | 100 kPa CO2, 6 h | 120 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 76 | [ |
| Entry | Catalystb | Zn form | Reaction condition | Light intensitya | Product | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | HPC-800 | Zn atom | 100 kPa CO2, 10 h | 300 mW•cm–2 | 4-Bromomethyl-1,3-dioxolan-2-one | 94 | [ |
| 2 | Zn SA-NC | Zn atom | 100 kPa CO2, 16 h | 300 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 99 | [ |
| 3 | ZNC-800 | Zn atom | 100 kPa CO2, 10 h | 1000 mW•cm–2 | 4-Methyl-1,3-dioxolan-2-one | 94 | [ |
| 4 | Zn-Asp-300 | ZnO | 100 kPa CO2, 4 h | 152.5 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 92 | [ |
| 5 | ZNPC-600 | ZnO | 100 kPa CO2, 6 h | 500 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 99 | [ |
| 6 | ZnS / NPC-2 | ZnS | 100 kPa CO2, 12 h | 300 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 98 | [ |
| 7 | ZnO/NC-L | ZnO | 100 kPa CO2, 6 h | 120 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 76 | [ |
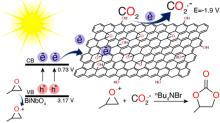
| Entry | Cathode|anode | Supporting electrolyte | Solvent | Reaction condition | Epoxide | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Ss|Mg | KBr, Ni(cyclam)Br2 | DMF | r.t., 100 kPa | Styrene oxide | 92 | [ |
| 2 | Cu|Mg/Al | [BMlm][BF4] | r.t., 100 kPa | Propylene oxide | 92 | [ | |
| 3 | CuNPs|Mg | 0.1 mol/L TEAI | MeCN | 25 ℃, 100 kPa | Propylene oxide | 86 | [ |
| 4 | Cu/CS|Mg | 0.1 mol/L TEAI | MeCN | r.t., 100 kPa | Propylene oxide | 94.7 | [ |
| 5 | AgNPs|Mg | 0.1 mol/L TEAI | MeCN | 25 ℃, 100 kPa | Propylene oxide | 70 | [ |
| 6 | Ss|Mg | TBAI | MeCN | r.t., 100 kPa | (R)-Styrene oxide | 59 (91% ee) | [ |
| 7 | C|Pt | TBAP, Ni(Ⅱ) complex | MeCN | r.t., 100 kPa | Styrene oxide | 100 | [ |
| 8 | Cu(ND)/CP|Pt | ZnCl2, TBAB | MeCN | r.t., 100 kPa | Styrene oxide | 74.9 | [ |
| 9 | HNSs|Pt | ZnCl2, TBAB | MeCN | r.t., 100 kPa | Styrene oxide | 66.9 | [ |
| 10 | Ti/TiO2-CNT-Pt|C | [APMIm]DCA | MeCN | 50 ℃, 100 kPa | Styrene oxide | 95 | [ |
| Entry | Cathode|anode | Supporting electrolyte | Solvent | Reaction condition | Epoxide | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Ss|Mg | KBr, Ni(cyclam)Br2 | DMF | r.t., 100 kPa | Styrene oxide | 92 | [ |
| 2 | Cu|Mg/Al | [BMlm][BF4] | r.t., 100 kPa | Propylene oxide | 92 | [ | |
| 3 | CuNPs|Mg | 0.1 mol/L TEAI | MeCN | 25 ℃, 100 kPa | Propylene oxide | 86 | [ |
| 4 | Cu/CS|Mg | 0.1 mol/L TEAI | MeCN | r.t., 100 kPa | Propylene oxide | 94.7 | [ |
| 5 | AgNPs|Mg | 0.1 mol/L TEAI | MeCN | 25 ℃, 100 kPa | Propylene oxide | 70 | [ |
| 6 | Ss|Mg | TBAI | MeCN | r.t., 100 kPa | (R)-Styrene oxide | 59 (91% ee) | [ |
| 7 | C|Pt | TBAP, Ni(Ⅱ) complex | MeCN | r.t., 100 kPa | Styrene oxide | 100 | [ |
| 8 | Cu(ND)/CP|Pt | ZnCl2, TBAB | MeCN | r.t., 100 kPa | Styrene oxide | 74.9 | [ |
| 9 | HNSs|Pt | ZnCl2, TBAB | MeCN | r.t., 100 kPa | Styrene oxide | 66.9 | [ |
| 10 | Ti/TiO2-CNT-Pt|C | [APMIm]DCA | MeCN | 50 ℃, 100 kPa | Styrene oxide | 95 | [ |
| [1] |
(a) Yu, B.; He, L. N. ChemSusChem 2014, 8, 52.
|
|
(b) Sakakura, T.; Choi, J.-C.; Yasuda, H. Chem. Rev. 2007, 107, 2365.
|
|
|
(c) Qiu, L. Q.; Yao, X. Y.; Zhang, Y. K.; Li, H. R.; He, L. N. J. Org. Chem. 2023, 88, 4942.
|
|
|
(d) Qiu, L. Q.; Li, H. R.; He, L. N. Acc. Chem. Res. 2023, 56, 2225.
|
|
| [2] |
Lang, X. D.; He, L. N. Chem. Rec. 2016, 16, 1337.
|
| [3] |
Prajapati, P. K.; Kumar, A.; Jain, S. L. ACS Sustainable Chem. Eng. 2018, 6, 7799.
|
| [4] |
Liu, C.; Niu, H.; Wang, D.; Gao, C.; Said, A.; Liu, Y.; Wang, G.; Tung, C.-H.; Wang, Y. ACS Catal. 2022, 12, 8202.
|
| [5] |
(a) Ahmed, S. H.; Bakiro, M.; Alzamly, A. Materialia 2020, 12, 100781.
|
|
(b) Bakiro, M.; Hussein Ahmed, S.; Alzamly, A. ACS Sustainable Chem. Eng. 2020, 8, 12072.
|
|
| [6] |
Cheng, R.; Wang, A.; Sang, S.; Liang, H.; Liu, S.; Tsiakaras, P. Chem. Eng. J. 2023, 466, 142982.
|
| [7] |
Gong, X.; Zhang, Y.; Xu, Y.; Zhai, G.; Liu, X.; Bao, X.; Wang, Z.; Liu, Y.; Wang, P.; Cheng, H.; Fan, Y.; Dai, Y.; Zheng, Z.; Huang, B. ACS Appl. Mater. Interfaces 2022, 14, 51029.
|
| [8] |
Jiang, H.; Zang, C.; Guo, L.; Gao, X. Sci. Total Environ. 2022, 838, 155920.
|
| [9] |
Kumar, A.; Samanta, S.; Srivastava, R. ACS Appl. Nano Mater. 2021, 4, 6805.
|
| [10] |
(a) Hussein Ahmed, S.; Bakiro, M.; Alzamly, A. Molecules 2021, 26, 1693.
|
|
(b) Bakiro, M.; Ahmed, S. H.; Alzamly, A. J. Environ. Chem. Eng. 2020, 8, 104461.
|
|
| [11] |
Zhai, G.; Liu, Y.; Lei, L.; Wang, J.; Wang, Z.; Zheng, Z.; Wang, P.; Cheng, H.; Dai, Y.; Huang, B. ACS Catal. 2021, 11, 1988.
|
| [12] |
Zhai, G.; Liu, Y.; Mao, Y.; Zhang, H.; Lin, L.; Li, Y.; Wang, Z.; Cheng, H.; Wang, P.; Zheng, Z.; Dai, Y.; Huang, B. Appl. Catal., B 2022, 301, 120793.
|
| [13] |
Das, R.; Manna, S. S.; Pathak, B.; Nagaraja, C. M. ACS Appl. Mater. Interfaces 2022, 14, 33285.
|
| [14] |
Zhang, H.; Si, S.; Zhai, G.; Li, Y.; Liu, Y.; Cheng, H.; Wang, Z.; Wang, P.; Zheng, Z.; Dai, Y.; Liu, T. X.; Huang, B. Appl. Catal., B 2023, 337, 122909.
|
| [15] |
Shi, Q.; Chen, M.-H.; Xiong, J.; Li, T.; Feng, Y.-Q.; Zhang, B. Chem. Eng. J. 2024, 481, 148301.
|
| [16] |
Das, A.; Mondal, R. K.; Chakrabortty, P.; Riyajuddin, S.; Chowdhury, A. H.; Ghosh, S.; Khan, A.; Ghosh, K.; Islam, S. M. Mol. Catal. 2021, 499, 111253.
|
| [17] |
Li, X.; Niu, X.; Fu, P.; Song, Y.; Zhang, E.; Dang, Y.; Yan, J.; Feng, G.; Lei, S.; Hu, W. Appl. Catal., B 2024, 350, 123943.
|
| [18] |
Saini, S.; Khan, S. R.; Gour, N. K.; Chandra Deka, R.; Jain, S. L. Green Chem. 2022, 24, 3644.
|
| [19] |
Yang, Q.; Yang, C. C.; Lin, C. H.; Jiang, H. L. Angew. Chem. Int. Ed. 2019, 58, 3511.
|
| [20] |
Gong, L.; Sun, J.; Liu, Y.; Yang, G. J. Mater. Chem. A 2021, 9, 21689.
|
| [21] |
Duan, C.; Ding, M.; Feng, Y.; Cao, M.; Yao, J. Sep. Purif. Technol. 2022, 285, 120359.
|
| [22] |
Liu, Y.; Chen, Y.; Liu, Y.; Chen, Z.; Yang, H.; Yue, Z.; Fang, Q.; Zhi, Y.; Shan, S. J. Catal. 2022, 407, 65.
|
| [23] |
Dai, W.; Zou, M.; Long, J.; Li, B.; Zhang, S.; Yang, L.; Wang, D.; Mao, P.; Luo, S.; Luo, X. Appl. Surf. Sci. 2021, 540, 148311.
|
| [24] |
Rong, W.; Ding, M.; Ma, P.; Kong, S.; Yao, J. J. Ind. Eng. Chem. 2024, 129, 682.
|
| [25] |
Tang, F.; Wang, L.; Ma, L.; Fang, Y.; Huang, J.; Liu, Y.-N. J. CO2 Util. 2021, 45, 101431.
|
| [26] |
Yang, Q.; Peng, H.; Zhang, Q.; Qian, X.; Chen, X.; Tang, X.; Dai, S.; Zhao, J.; Jiang, K.; Yang, Q.; Sun, J.; Zhang, L.; Zhang, N.; Gao, H.; Lu, Z.; Chen, L. Adv. Mater. 2021, 33, 2103186
|
| [27] |
Wang, Y.; Liu, H.; Shi, Q.; Miao, Z.; Duan, H.; Wang, Y.; Rong, H.; Zhang, J. Angew. Chem. Int. Ed. 2024, e202404911.
|
| [28] |
Paliwal, K. S.; Sarkar, D.; Mitra, A.; Mahalingam, V. ChemPlus-Chem 2023, 88, e202300448.
|
| [29] |
Wang, T.; Chen, F.; Jiang, L.; Li, J.; Chen, K.; Gao, J. Inorg. Chem. 2024, 63, 4224.
|
| [30] |
Guo, Q.; Xia, S.-G.; Li, X.-B.; Wang, Y.; Liang, F.; Lin, Z.-S.; Tung, C.-H.; Wu, L.-Z. Chem. Commun. 2020, 56, 7849.
|
| [31] |
Yang, Z.; Xie, Y.; Feng, Y.; Yao, J. J. Environ. Chem. Eng. 2024, 12, 112310.
|
| [32] |
(a) Chen, H.; Fan, L.; Zhang, X. ACS Appl. Mater. Interfaces 2020, 12, 54884.
|
|
(b) Yin, M.; Wang, L.; Tang, S. ACS Catal. 2023, 13, 13021.
|
|
|
(c) Ding, M.; Jiang, H.-L. ACS Catal. 2018, 8, 3194.
|
|
| [33] |
Sharma, N.; Dhankhar, S. S.; Nagaraja, C. M. Microporous Mesoporous Mater. 2019, 280, 372.
|
| [34] |
Zhou, X.; Zhang, H.; Cheng, H.; Wang, Z.; Wang, P.; Zheng, Z.; Dai, Y.; Xing, D.; Liu, Y.; Huang, B. J. Colloid Interface Sci. 2024, 658, 805.
|
| [35] |
Fang, Z.; Deng, Z.; Wan, X.; Li, Z.; Ma, X.; Hussain, S.; Ye, Z.; Peng, X. ppl. Catal., B 2021, 296, 120329.
|
| [36] |
Duan, C.; Xie, Y.; Ding, M.; Feng, Y.; Yao, J. J. CO2 Util. 2022, 64, 102158.
|
| [37] |
Feng, Y.; Cao, Y.; Zhu, J.; Han, H.; Liu, Y.; Li, X.; Zhao, S.; Yang, J.; Fang, Z.; He, W.; Yang, Z.; Guo, K. J. Cleaner Prod. 2024, 440, 141002.
|
| [38] |
Ding, L.-G.; Yao, B.-J.; Wu, W.-X.; Yu, Z.-G.; Wang, X.-Y.; Kan, J.-L.; Dong, Y.-B. Inorg. Chem. 2021, 60, 12591.
|
| [39] |
Zhang, L.; Tu, X.; Chen, Y.; Han, W.; Chen, L.; Sun, C.; Zhu, S.; Song, Y.; Zheng, H. Mol. Catal. 2023, 538, 112971.
|
| [40] |
(a) Wu, Y.; Yu, X.-F.; Du, Y.; Xia, L.; Guo, Q.; Zhang, K.; Zhang, W.; Liu, S.; Peng, Y.; Li, Z.; Yang, X. Appl. Catal., B 2023, 331, 122732.
|
|
(b) Zhang, W.; Li, Z.; Yu, X.-F.; Zhang, K.; Liu, S.; Du, Y.; Guo, Q.; Zhang, L.; Ding, X.; Tang, H.; Peng, Y.; Yang, X. Inorg. Chem. 2024, 63, 2954.
|
|
| [41] |
Tascedda, P.; Weidmann, M.; Dinjus, E.; Duach, E. Appl. Organomet. Chem. 2001, 15, 141.
|
| [42] |
Yang, H.; Gu, Y.; Deng, Y.; Shi, F. Chem. Commun. 2002, 274.
|
| [43] |
Wu, L.-X; Yang, H.-P; Wang, H.; Lu, J.-X. RSC Adv. 2015, 5, 23189.
|
| [44] |
Zhang, J.-J.; Shan, S.-L.; Shi, Y.; Hou, Y.; Wang, H.; Lu, J.-X. J. Electroanal. Chem. 2021, 882, 114962.
|
| [45] |
Wu, L.-X.; Yang, H.-P.; Guan, Y.-B.; Yang, M.-P.; Wang, H.; Lu, J.-X. Int. J. Electrochem. Sci. 2017, 12, 8963.
|
| [46] |
Xiao, Y.; Chen, B.-L.; Yang, H.-P.; Wang, H.; Lu, J.-X. Electrochem. Commun. 2014, 43, 71.
|
| [47] |
Khoshro, H.; Zare, H. R.; Namazian, M.; Jafari, A. A.; Gorji, A. Electrochim. Acta 2013, 113, 263.
|
| [48] |
Li, W.-Z; Qi, K.; Lu, X.-Y; Qi, Y.; Zhang, J.-L; Zhang, B.-S; Qi, W. Chem.-Eur. J. 2022, 28, e202200622
|
| [49] |
Dai, X.-Y; Qi, K.; Liu, C.-W; Lu, X.-Y; Qi, W. Carbon 2023, 202, 51.
|
| [50] |
Hu, Y. -L.; Liu, X. -B.; Rong, Q. Green Chem. Lett. Rev. 2023, 16, 2163192.
|
| [51] |
Gao, X.-F; Yuan, G.-Q; Chen, H.-J; Jiang, H.-F; Li, Y.-W; Qi, C-R. Electrochem. Commun. 2013, 34, 242.
|
| [52] |
Wang, H; Wu, L.-X; Lan, Y.-C; Zhao, J.-Q; Lu, J.-X. Int. J. Electrochem. Sci. 2011, 6, 4218.
|
| [53] |
Wu, L. -X; Wang, H; Tu, J -Z.-Y; Ding, B. -B; Xiao, Y; Lu, J.-X. Int. J. Electrochem. Sci. 2012, 7, 11540.
|
| [54] |
Wang, H.; Wu, L.-X.; Zhao, J.-Q.; Li, R.-N.; Zhang, A.-J.; Kajiura, H.; Li, Y.-M.; Lu, J.-X. Greenhouse Gas. Sci. Technol. 2012, 2, 59.
|
| [55] |
(a) Liu, J.; Yang, G.; Liu, Y.; Wu, D.; Hu, X.; Zhang, Z. Green Chem. 2019, 21, 3834.
|
|
(b) Iglesias, D.; Tinajero, C.; Marchetti, S.; Roppolo, I.; Zanatta, M.; Sans, V. Green Chem. 2023, 25, 9934.
|
|
| [56] |
(a) Chung, M.; Maalouf, J. H.; Adams, J. S.; Jiang, C.; Román-Leshkov, Y.; Manthiram, K. Science 2024, 383, 49.
pmid: 32527828 |
|
(b) Leow, W. R.; Lum, Y.; Ozden, A.; Wang, Y.; Nam, D.-H.; Chen, B.; Wicks, J.; Zhuang, T.-T.; Li, F.; Sinton, D.; Sargent, E. H. Science 2020, 368, 1228.
doi: 10.1126/science.aaz8459 pmid: 32527828 |
|
|
(c) Tao, Y.; Huang, C.; Lu, Q. Nat. Catal. 2023, 6, 1107.
pmid: 32527828 |
|
|
(d) Yang, Y.; Yuan, X.; Wang, Q.; Wan, S.; Lin, C.; Lu, S.; Zhong, Q.; Zhang, K. Angew. Chem. Int. Ed. 2024, 63, e202314383.
pmid: 32527828 |
|
|
(e) Seitz, A. K.; Kohlpaintner, P. J.; van Lingen, T.; Dyga, M.; Sprang, F.; Zirbes, M.; Waldvogel, S. R.; Gooßen, L. J. Angew. Chem. Int. Ed. 2022, 61, e202117563.
pmid: 32527828 |
|
| [57] |
Zhang, J.-J.; Li, S.-M.; Shi, Y.; Hu, Q.-L.; Wang, H.; Lu, J.-X. New J. Chem. 2020, 44, 11817.
|
| [1] | 夏坤, 张开发, Sher Wali Khan, 阿布力米提•阿布都卡德尔. 二氧化碳参与的三组分偶联反应进展[J]. 有机化学, 2024, 44(5): 1506-1525. |
| [2] | 段东森, 马媛, 刘宇博, 程富, 朱道勇, 王少华. 可见光诱导的二氧化碳对活化烯烃的脱碳羧基化反应[J]. 有机化学, 2024, 44(5): 1675-1685. |
| [3] | 姜晓琳, 王超洋, 武利园, 李跃辉. 含咔唑结构的小分子及聚合物催化二氧化碳转化研究进展[J]. 有机化学, 2024, 44(5): 1423-1444. |
| [4] | 吕帅, 朱钢国, 姚金忠, 周宏伟. 电化学介导的氧化羧化及二氧化碳还原羧化制备羧酸的研究进展[J]. 有机化学, 2024, 44(3): 780-808. |
| [5] | 侯静, 黄燕, 李浩, 万远翠, 邵雨, 詹乐武, 王定海, 李斌栋. 二氧化碳自由基阴离子的应用研究进展[J]. 有机化学, 2024, 44(10): 3117-3135. |
| [6] | 李嘉元, 易雅平, 席婵娟. 二氧化碳参与的芳香化合物去芳构化羧化反应研究进展[J]. 有机化学, 2024, 44(10): 3136-3146. |
| [7] | 石亲, 李臻, 何林, 李玉东, 李跃辉. 硼促进Co催化使用CO2和H2实现仲芳香胺N-甲基化[J]. 有机化学, 2024, 44(10): 3233-3240. |
| [8] | 袁盼锋, 朱灿明, 孟庆元. 光化学转化二氧化碳合成羧酸化合物的研究进展[J]. 有机化学, 2024, 44(10): 2997-3042. |
| [9] | 徐辉, 蒋慧娴, 阚磊, 徐佩, 朱旭. 可见光诱导甲酸盐参与的炔烃氢羧基化反应[J]. 有机化学, 2024, 44(10): 3241-3248. |
| [10] | 李建文, 王涛, 陶晟, 陈飞, 李敏, 刘宁. SBA-15负载的N-杂环卡宾-吡啶钼配合物在二氧化碳转化制备环状碳酸酯中的应用[J]. 有机化学, 2024, 44(10): 3213-3222. |
| [11] | 张誉元, 杨昌杰, 唐海涛, 潘英明. 异相催化固定二氧化碳合成羰基衍生物的研究进展[J]. 有机化学, 2024, 44(10): 3077-3090. |
| [12] | 高小童, 钟昱卿, 冯楠, 孙莹, 杨得勇, 周锋. 惰性键与二氧化碳的电化学羧化反应研究[J]. 有机化学, 2024, 44(10): 3043-3062. |
| [13] | 赵盈喆, 张子瑄, 张建玲, 张仁杰, 李美玲, 滕钰楠, 王昊翔. 铈掺杂锆基金属-有机框架纳米颗粒的制备及其光催化二氧化碳环加成反应催化性能研究[J]. 有机化学, 2024, 44(10): 3169-3177. |
| [14] | 李文珂, 孙北奇, 张雷, 莫凡洋. 基于自由基机理光催化羧基化反应研究进展[J]. 有机化学, 2024, 44(10): 2961-2996. |
| [15] | 张澳龙, 杨晗, 程佩栋, 姚阳, 孙松. 可见光促进烯烃与丙二酸酯、CO2的碳-羧化反应研究[J]. 有机化学, 2024, 44(10): 3159-3168. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||